Summary
In a meta-analysis that was published in 2005, Huxley et al. reported an almost 2-fold higher risk for pancreatic cancer that was associated with type 2 diabetes mellitus (T2DM; OR, 1.82; 95% CI, 1.66 to 1.89) [Br J Cancer 2005]. There was also a similar, strong relationship between obesity (body mass index >30 kg/m2) and cancer, which leads to the question of whether the cancer risk stems from the diabetes or if is it related to obesity.
- Gastrointestinal Cancers
- Diabetes Mellitus
In a meta-analysis that was published in 2005, Huxley et al. reported an almost 2-fold higher risk for pancreatic cancer that was associated with type 2 diabetes mellitus (T2DM; OR, 1.82; 95% CI, 1.66 to 1.89) [Br J Cancer 2005]. There was also a similar, strong relationship between obesity (body mass index >30 kg/m2) and cancer, which leads to the question of whether the cancer risk stems from the diabetes or if is it related to obesity. Edwin Gale, MD, University of Bristol, Bristol, United Kingdom, provided an update on the relationship between cancer and diabetes, and the impact of potential therapies on cancer risk in diabetic patients.
Worldwide, pancreatic cancer is the fourth most common cause of cancer-related mortality [www.seer.cancer.gov]. Prognosis is poor, with only a 5% to 10% survival rate at 5 years [Hariharan D et al. HBP 2008]. In the United States, pancreatic cancer was the fourth most lethal cancer in 2010, after lung, colorectal, and breast cancer [www.pancan.org]. Risk factors may be either inherited (eg, familial, genetic syndromes, germline mutations, having a non-O blood type, being of African-American ethnic origin) or acquired (eg, chronic pancreatitis, cigarette smoking, obesity, T2DM). Pancreatic cancer presents at an advanced state, at which point it is typically 50% metastasized.
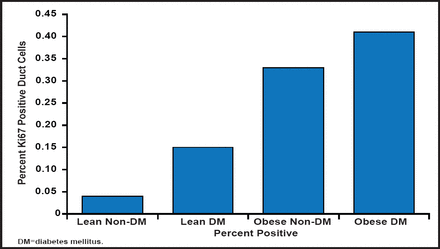
Does cancer cause diabetes or vice versa? Prof. Gale discussed possible explanations for the relationship. Is the relationship genetic or environmental? While either is a possibility, the most reasonable conclusion is that it is environmental, as the relationship seems to be mostly mediated by obesity (insulin resistance). Obesity and diabetes are both associated with increased pancreatic duct replication, a predisposing factor for pancreatitis and pancreatic cancer. This suggests that increased low-grade pancreatic inflammation is potentially a factor in the generation of cancer in these two conditions (Figure 1) [Butler AE et al. Diabetologia 2010].
Association Between Obesity, Diabetes, and Increased Pancreatic Duct Replication.
Reproduced with permission from E Gale, MD.
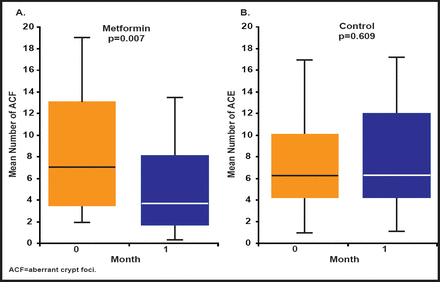
Of the two diabetes-specific factors (hyperglycemia and diabetes therapies), diabetes therapies have provoked the most interest as either causative or protective. Two studies have shown that the use of metformin is associated with a lower risk of cancer of the colon or pancreas (although it does not affect the risk of breast or prostate cancer; Figure 2) [Currie CJ et al. Diabetologia 2009; Li D et al. Gastroenterology 2009]. Finally, there are tumor-specific factors. Pancreatic cancer has been shown to promote insulin resistance and therefore can precipitate new diabetes or aggravate preexisting disease [Li D et al. Cancer Cause Control 2011].
Pancreatic Cancer and Diabetes Therapy.
Reproduced with permission from E Gale, MD.
Despite the seriousness of this relationship, Prof. Gale does not advocate screening for pancreatic cancer in newly diagnosed diabetic patients, as there are no reliable markers of early pancreatic cancer or a specific marker for pancreatic cancer-associated diabetes. Clinically, we should now consider the possibility of pancreatic cancer when there is a rapid/unexplained progression or loss of glucose control. There is an immediate need to investigate potential hyperglycemic factors and mechanisms of insulin resistance, or possible β-cell dysfunction, in carcinoma of the pancreas, which could lead to better diagnostic markers.
The relationship between cancer and diabetes mortality rates was first studied in 1914, when neither was a major public health problem [Greenwood Jr. M & Wood F. J Hygiene 1914]. Now, both are increasing public health concerns worldwide. Michael Pollak, MD, McGill University, Montreal, Quebec, Canada, discussed the need to clarify the associations between these diseases, which are increasing in parallel around the world. The new and growing area of concern is the relationship between insulin treatment and cancer, which may have a major impact on the viability of new drug development projects and also on prescribing practices for existing agents.
The current view suggests that it is plausible that insulin+ impaired fasting glucose (IGF) signaling has roles beyond those that are now recognized as medically relevant. It is known that cancer cells are potential targets for the various hormones and cytokines that are elevated in T2DM and that cancers can have varying sensitivity or resistance to endogenous insulin or other hormones and cytokines that are associated with T2DM. Increased body weight is associated with increased death rates for all cancers combined and for cancers at multiple specific sites [Calle EE et al. N Engl J Med 2003; Ma J et al. Lancet Oncol 2008]. However, it is the molecular characteristics of a tumor that determine the influence of the host nutritional status—not the level of dietary restriction or excess.
A recent surprise and much discussed finding is that metformin use may be associated with a reduced risk of cancer. Metformin itself was never optimized for oncological indications with respect to pharmacokinetics, however, and it is not clear if there are implications for nondiabetic subjects. As an oncologist, Prof. Pollack feels that there is an urgent need to clarify these data. “If this is true, this represents one of largest, most effective treatments for cancer we have ever seen.”
Metformin is prescribed to 120 million people in the world for the treatment of T2DM. Recent research shows that metformin reduces the risk of cancer in T2DM patients. Frédéric Bost, PhD, INSERM, Nice, France, discussed the recent research that has demonstrated the cancer-preventing properties of metformin, along with an explanation of the cellular and molecular mechanisms of action of metformin on cancer cells.
Metformin acts on the liver by decreasing glucose production, on adipose tissue by increasing glucose transport and decreasing lipolysis, and on muscle by increasing fatty acid oxidation and glucose transport. All of these actions result in a decrease in hyperglycemia and insulinemia.
Preclinical studies have shown that oral and intraperitoneal treatment with metformin leads to a 50% and 35% reduction in tumor growth, respectively, in mice that bear xenografts of LNCaP [Ben Sahra I et al. Oncogene 2008], while metformin significantly reduces the growth of MIAPaCa-2 and PANC-1 cells that have been xenografted onto the flank of nude mice, suggesting that metformin could be a potential candidate in novel treatment strategies for human pancreatic cancer [Kisfalvi K et al. Cancer Res 2009]. Metformin prevents lung carcinogenesis and improves the antitumoral effects of chemotherapeutic agents in mice. Combined with doxorubicin, it completely inhibited breast cancer cell growth in mice and cancer stem cells in the tumor.
More than 20 ongoing trials that are using metformin can be found at www.clinicaltrials.gov. The first trial of this nature reported that metformin inhibited colorectal carcinogenesis in humans by suppressing colonic epithelial proliferation and aberrant rectal crypt foci formation [Hosono K et al. Cancer Prev Res 2010].
Prof. Bost suggested that metformin could be acting indirectly via a paracrine or endocrine effect or directly on cancer cells. Insulin is a growth factor that can promote tumor growth, which can be reversed by metformin [Algire C et al. Endocr Relat Cancer 2010]. Metformin may also act by affecting the cells of the tumor microenvironment and interfering with inflammation by way of TNF-α [Pearce El et al. Nature 2009]. There are also studies that show that metformin directly affects cancer cells. It has been demonstrated that metformin acts as a growth inhibitor for epithelial cells by means of AMP kinase pathway activation, as growth inhibition is associated with decreased mammalian target of rapamycin and S6 kinase activation and a general decrease in mRNA translation [Zahikihani M et al. Cancer Res 2006].
Other suggested cellular and molecular mechanisms of action of metformin on cancer cells include the promotion of cell cycle arrest [Ben Sahra I et al. Oncogene 2008], induction of apoptosis [Zhuang Y et al. Mol Cancer Res 2011], and the inhibition of oxidative phosphorylation [Ben Sahra I et al. Cancer Res 2010].
Metformin exhibits a strong and consistent antiproliferative effect on several cancer cell lines, including breast, colon, ovarian, pancreatic, lung, and prostate cancer cells. “In the next couple of years, the results of the numerous ongoing clinical trials should help to determine how to best use metformin in cancer therapy,” concluded Prof. Bost.

The editors would like to thank the many members of the European Association for the Study of Diabetes 2011 Annual Meeting presenting faculty who generously gave their time to ensure the accuracy and quality of the articles in this publication.
- © 2011 MD Conference Express®