Summary
Investigators in the ALPHA OMEGA Trial [NCT00127452] concluded that that low doses of n-3 fatty acids, given in the form of enriched margarines, do not reduce major cardiovascular events.
- Cardiology Clinical Trials
- Prevention & Screening
Investigators in the ALPHA OMEGA Trial (NCT00127452) concluded that that low doses of n-3 fatty acids, given in the form of enriched margarines, do not reduce major cardiovascular (CV) events. The results of the trial were presented by Daan Kromhout, MD, Wageningen University, Wageningen, The Netherlands, and were in accordance with those of prior studies by Saravanan and colleagues [Saravanan P et al. Lancet 2010]. They concluded that the effect of n-3 fatty acids diminished with increasing drug treatment of CV risk factors and that no effect was observed in state-of-the-art-treated patients in the most recent trial.
The ALPHA OMEGA Trial was designed to examine the effects of low doses of the n-3 fatty acids eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and/or ALA in margarine (targeted average intake = 20 g/day) in stable Dutch post-MI (≤10 years) patients (n=4837; 78% men). Subjects (mean age 69 years; range 60 to 80 years) were randomly assigned to receive: EPA-DHA placebo+ALA placebo (n=1236), 400 mg EPA-DHA+ALA placebo (n=1192), EPA-DHA placebo+2 g ALA (n=1197), or 400 mg EPA-DHA+2 g ALA (n=1212) and were followed for 40 months.
The primary study outcome was major CV events. Important secondary endpoints were fatal coronary heart disease and ventricular arrhythmia-related events, defined as sudden death, cardiac arrest, and cardioverter-defibrillator placement.
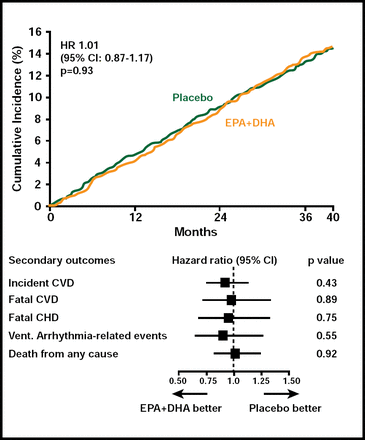
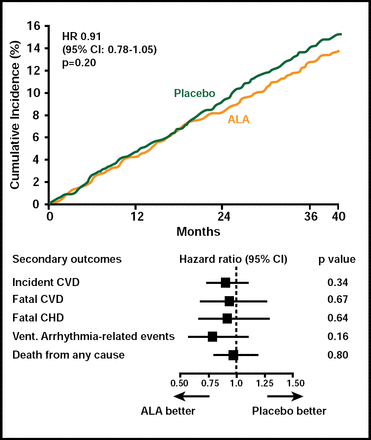
A total of 671 patients developed a major CV event. There was no difference in either the primary or secondary outcomes between subjects who received EPA+DHA or ALA compared with those who received placebo (Figures 1 & 2). Among subgroups, however, there was a nonsignificant (HR, 0.73; 95% CI, 0.51 to 1.03; p=0.07) 27% reduction in the primary endpoint among women who received ALA. In an exploratory analysis, diabetic subjects (n=1014) who received EPA+DHA showed a 49% (HR, 0.51; 95% CI, 0.27 to 0.97) reduction in coronary heart disease mortality and ventricular arrhythmia-related events (HR, 0.51; 95% CI, 0.24 to 1.11). Subjects who received ALA had a 61% reduction in ventricular arrhythmia-related events (HR, 0.39; 95% CI, 0.17 to 0.88). Adverse events did not differ among the study groups.
EPA+DHA Endpoints.
Reproduced with permission from D. Kromhout, MD.
ALA Endpoints.
Reproduced with permission from D. Kromhout, MD.
Commenting on the differences between the ALPHA OMEGA Trial and earlier trials, Luigi Tavazzi, MD, Villa Maria Cecilia Hospital, Cotignola, Italy, noted three factors that may have influenced the results: the relatively small sample size, the low dose of EPA-DHA, and whether the components of the composite primary endpoint were specific enough for the specific mechanisms of action of n-3 fatty acids. Of note, the endpoints in question were based on previous prospective cohort studies that demonstrated that n-3 fatty acids lowered the risk of coronary heart disease as well as stroke. While praising the investigators for attempting the trial, Prof. Tavazzi pointed out that clinicians should use caution when using the ALPHA OMEGA Trial results to draw conclusions about the effect of n-3 fatty acids in this studied population.
Results of the ALPHA OMEGA Trial were published online in the New England Journal of Medicine at NEJM.org (10.1056/NEJMoa1003603).
- © 2010 MD Conference Express