Summary
This article discusses long-term results from the MULTIcentre evaluation of Single high-dose bolus TiRofiban versus Abciximab with sirolimus-eluting sTEnt or Bare Metal Stent in Acute Myocardial Infarction study [MULTI STRATEGY; NCT00229515].
- Interventional Techniques & Devices Myocardial Infarction
Long-term results from the MULTIcentre evaluation of Single high-dose bolus TiRofiban versus Abciximab with sirolimus-eluting sTEnt or Bare Metal Stent in Acute Myocardial Infarction study (MULTI STRATEGY; NCT00229515), presented by Marco Valgimigli, MD, University of Ferrara, Ferrara, Italy, show sustained efficacy for sirolimus-eluting stents (SES) over bare metal stents (BMS) in reducing target vessel revascularization (TVR), with no difference in death, repeat myocardial infarction (MI), or overall stent thrombosis in a broad population of subjects who were undergoing angioplasty for ST-elevation myocardial infarction (STEMI). Tirofiban was shown to be noninferior to abciximab.
MULTI STRATEGY was a multicenter, 2×2 factorial, randomized, phase IV study that was designed to investigate whether the use of tirofiban, given at high-dose bolus, resulted in clinical outcomes that were similar to those achieved with abciximab and to evaluate the long-term safety/efficacy profile of SES over BMS in STEMI patients. Subjects included all-comer STEMI patients who were randomized before arterial sheath insertion to receive either tirofiban (bolus of 25 μg/kg, followed by an 18- to 24-hour infusion at 0.15 μg/kg/min) or abciximab. Four drug/stent combinations were studied: tirofiban+ SES (n=186), tirofiban+BMS (n=186), abciximab+SES (n=187), and abciximab+BMS (n=186).
The primary study outcome for the drug comparison was ≥50% ST-segment elevation resolution on a noninferiority basis. For the stent comparison, the primary endpoint was the rate of major adverse cardiac events (MACEs), defined as the composite of death from any cause, reinfarction, and clinically driven TVR within 8 months. The primary stent results were published previously [Valgimigli M et al. JAMA 2008].
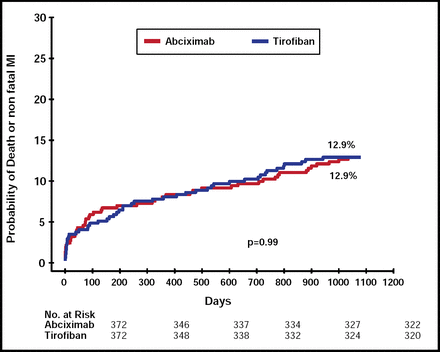
Data for 99% of the study participants (736 patients) were presented. At 3 years, all-cause mortality was 6.7% in the tirofiban group and 7.8% in the abciximab group (p=0.56) and 7.5% in the BMS versus 7.0% in the SES groups (p=0.79). The composite of all-cause death or MI was 12.9% in both groups (p=0.99; Figure 1) and 13.2% in the BMS versus 12.6% in the SES groups (p=0.83). The results were consistent among prespecified subgroups, including age, gender, diabetes status, and stent type. The overall rate for death, MI, or TVR at 3 years remained significant (p=0.026) between the two stent groups (15.9% for SES vs 22.3% for BMS).
3-Year Outcomes: All-Cause Death or MI.
Reproduced with permission from M. Valgimigli, MD.
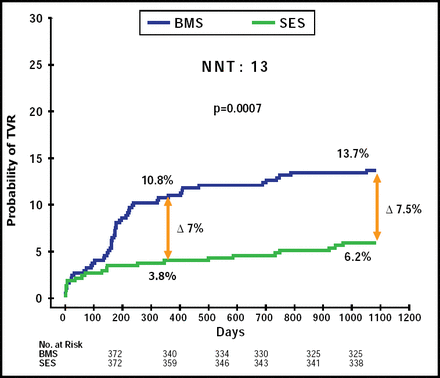
The need for TVR was twice as common with BMS (13.7% vs 6.2% with SES. HR, 2.29; 95% CI, 1.4 to 3.7; p=0.0007; Figure 2). The rate of definite ST was 3.5% in both groups (p>0.99); the cumulative incidence of definite or probable ST was 4.0% in the SES group and 4.6% in the BMS group (p=0.71), whereas the cumulative incidence of definite, probable, or possible ST was 6.2% versus 5.1% for SES versus BMS, respectively (p=0.55).
Probability of TVR.
Reproduced with permission from M. Valgimigli, MD.
- © 2010 MD Conference Express