Summary
Postpericardiotomy syndrome (PPS), a complication that often follows cardiac surgery, occurs in 10% to 45% of patients, and though some treatment approaches may be used, optimal treatment for PPS prevention has yet to be established [Finkelstein Y et al. Herz 2002]. This article discusss results from the COlchicine for the Prevention of the Postpericardiotomy Syndrome [COPPS; NCT00128427].
- Cardiology Clinical Trials
- Interventional Techniques & Devices
Colchicine therapy is safe and effective for the prevention of postpericardiotomy syndrome (PPS) and may decrease the risk of postsurgical PPS development by >50%. PPS, a complication that often follows cardiac surgery, occurs in 10% to 45% of patients, and though some treatment approaches, such as NSAIDs, colchicine, and corticosteroids, may be used, optimal treatment for PPS prevention has yet to be established [Finkelstein Y et al. Herz 2002]. Massimo Imazio, MD, Maria Vittoria Hospital, Torino, Italy, discussed results from the COlchicine for the Prevention of the Postpericardiotomy Syndrome (COPPS; NCT00128427).
COPPS was a multicenter, double-blind study that included 360 patients who were randomized to colchicine (n=180; 1.0 mg twice daily for 1 day followed by 0.5 mg twice daily for 1 month for patients ≥70 kg or 0.5 mg twice daily for 1 day followed by 0.5 mg for 1 month for patients <70 kg) or placebo (n=180) on the third postoperative day. PPS was defined as the presence of at least two of the following criteria: fever that lasted beyond the first postoperative week without evidence of systemic or focal infection, pleuritic chest pain, friction rub, pleural effusion, and new or worsening pericardial effusion. The primary efficacy endpoint was the incidence of PPS at 12 months, and the secondary endpoint was the combined rate of disease-related hospitalization, cardiac tamponade, constrictive pericarditis, and relapses. The groups were well matched at baseline.
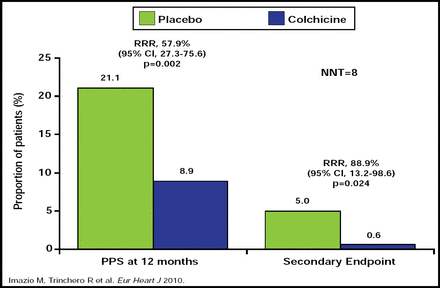
At 12 months, there was a significant reduction in the incidence of PPS among patients who were treated with colchicine compared with placebo (RRR, 57.9%; 95% CI, 27.3 to 75.6; p=0.002; NNT=8; Figure 1). The rate of the composite secondary endpoint was also lower in the colchicine group compared with placebo (0.6% vs 5.0%, respectively; p=0.024). The adverse event profiles were similar for both groups, with no severe side effects reported across the study population. The most common side effects were gastrointestinal in nature for both groups.
COPPS Trial: Main Results.
Reproduced with permission from Oxford University Press.
This study demonstrated that colchicine halves the risk of PPS following cardiac surgery compared with placebo. This therapeutic strategy appears to be safe and effective for the prevention of postsurgical PPS. It is important to note that the diagnostic criteria for PPS were nonspecific and allowed for the detection of milder forms of pleuropericardial involvement following cardiac surgery, because at present, there are no guidelines or consensus documents on the diagnosis of PPS. Therefore, further study is warranted to determine the strength of these data.
- © 2010 MD Conference Express