Summary
It is increasingly an open question as to when and where point-of-care (POC) glucose meters should be used and what clinical decisions should be made based on POC readings. This article discusses related issues in a dedicated lecture on the subject, as well as implications for for tight glucose control, currently the standard of care in intensive care units.
- Hyperglycemia/Hypoglycemia
- Insulin
It is increasingly an open question as to when and where point-of-care (POC) glucose meters should be used and what clinical decisions should be made based on POC readings. In one recent study, five of the most commonly used POC devices were compared and found to have an average percentage difference between monitor pairs that was statistically significant in more than half of the paired comparisons (p<0.05), with significant differences ranging from 5.7% to 32.0% [Kimberly MM et al. Clin Chim Acta 2006]. Such broad variability is of greatest concern in the hospital setting, where patients, particularly those who are critically ill, will exhibit those confounders, such as anemia, that further skew the accuracy of a given POC device measure. As reviewed by Dungan et al., the observed error rates are high enough to lead to missed or overreaching medical interventions that may result in patient suffering and possibly death [Dungan K et al. Diabetes Care 2007].
Mitchell Scott, PhD, Washington University School of Medicine, St. Louis, MO, and Richard Hellman, MD, FACP, FACE, University of Missouri-Kansas City School of Medicine, Kansas City, MO, both addressed this issue in a dedicated lecture on the subject. Dr. Scott explained the implications for for tight glucose control (TGC), currently the standard of care in intensive care units.
Dr. Scott noted that, as of 2008, there were at least 30 POC meters on the market, representing a $6 billion-a-year health care investment. Recent innovations have brought so-called “no wipe” strips, sample volume detection, smaller sample sizes, faster analysis, and data storage and capture. However, in contrast to these advances, and despite doctor/patient enthusiasm regarding the availability of simple-to-use, portable meters, significant errors occur due to user error or as a result of the intrinsic nature of the device.
For many devices, common interferences include the effect of aberrant hematocrit levels. Anemic patients may register higher blood glucose values than actual, or polycythemia may result in measures that are lower than actual values. Meter readings may also be skewed by reducing agents, such as ascorbate or acetaminophen. Problems are also common with test strips that rely on some glucose dehydrogenase detection methods (GDH-PQQ) [Dungan KM et al. Diabetes Care. 2007]. This last issue was serious enough to prompt the FDA to advise the avoidance of GDH-PQQ glucose test strip use in health care facilities in August of 2009.
Interferences that are problematic in homecare may be life-threatening in the critical care setting. Anemic hematocrit levels <30 are not unusual in the ICU, nor is the administration of intravenous immunoglobulin (IVIG), which contains a maltose component, or icodextrin, which is present in peritoneal dialysis solution. Both compounds would be read as glucose by GDH-PQQ strip POC meters.
POC in the ICU
In a landmark paper in 2001, van den Berghe and colleagues established that tight glycemic control (TGC; <130 mg/dL) in critically ill patients saves lives [van den Berghe G et al. N Engl J Med 2001]. This conclusion led to the adoption of TGC in medical institutions all over the world and a subsequent increase in critical care POC meter testing. At Dr. Scott's institution, the Barnes-Jewish Hospital, the use of test strips grew from a quarter of a million strips in 2000 to over half a million strips by 2009. A central lab can not process samples fast enough for the real-time insulin adjustments of TGC. Therefore, POC must be used.
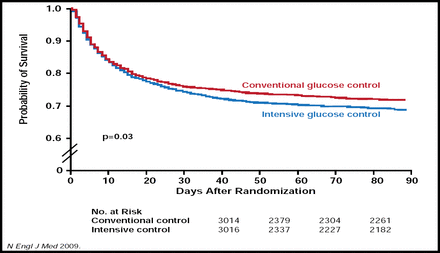
The health benefits of the TGC protocol in practice were evaluated in many small studies, but not until 2008 did a meta-analysis of 27 studies of TGC determine that not only was there no benefit in morbidity and mortality for critically ill patients but a 3–5-fold increased risk of hypoglycemia had been introduced to study subjects [Wiener RS et al. JAMA 2008]. One year later, results from the randomized NICE-SUGAR trial (n=6104) found a decreased rate of survival and an increased incidence of hypoglycemia in patients who were undergoing TGC (Figure 1) [NICE-SUGAR Study Investigators. N Engl J Med 2009].
NICE-SUGAR. One-Year Survival Rates.
Copyright © 2009 Massachusetts Medical Society. All rights reserved.
Why were so many patients not seeing the benefits of van den Berghe's protocol? The answer, Dr. Scott proposed, may lie in part in the methods of glucose testing. Van den Berghe and colleagues used arterial gas analyzers. Of the 27 studies in the meta-analysis, only 10 actually reported the method of testing, and of those, 8 used POC meters. In the NICE-SUGAR study, the method of glucose testing varied by participating institution and POC meters were allowed. These combined results led Dr. Scott to suggest in his published commentary that currently available POC glucose meters may not be adequate for TCG protocols until measuring standards can be improved. “If it's a given that meters are not used for diagnosis, should they really be used for dosing intravenous insulin [in critical care]?” [Scott MG et al. Clin Chem 2009].
Dr. Scott reviewed the allowable error rates for meters and those being commonly observed in practice. The Clinical Laboratory Improvement Act allows for an error rate of 10% or 6 mg/d, whichever is greater. The ADA suggests <5% while the FDA allows for a 20% error rate at values of >100 mg/dL or 12 mg/dL at <100 mg/dL [Chen E et al. Diab Tech Ther 2003]. The ADA criteria seem most ideal; however, Dr. Scott acknowledged that no currently available meter can achieve such accuracy.
Investigations that have compared metered values to the central laboratory or to the reading of other meters indicate a broad range of accuracy. A study by the Center for Disease Control (CDC), evaluating 5 commonly used meters, in which a single technician tested 93 subjects at 12 samples each, showed an error rate of 11% relative to laboratory values and a difference as high as a 32% in values recorded between meters [Kimberly MM et al. Clin Chim Acta 2006]. A study from Johns Hopkins showed error rates as high as 8.7% [Chen E et al. Diab Tech Ther 2003].
Clinical Dilemma
Dr. Hellman pointed out that rapid testing time and convenience of POC meters have greatly enhanced the clinician's ability to adjust insulin levels for patients whose need is urgent. However, though results within allowable rates of error are considered a reasonable tradeoff by some, Dr. Hellman sees considerable danger in statistical outliers (those glucose results that deviate by relatively large values from the true reference glucose value).
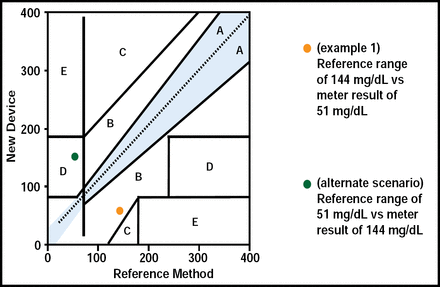
Consider a recent case in which a 71-year-old man, with a diabetes duration of 24 years, currently on insulin infusion, presented with fever, chills, a blood pressure of 74/40 mm Hg, and glucose measure of 51 mg/dL by POC meter. The patient was admitted to the ICU, insulin infusion was discontinued, and IV glucose was initiated. Central lab measures subsequently revealed a glucose level of 144 mg/dL. “The patient had been improperly treated,” said Dr. Hellman. POC meters are known to give false readings in patients who are hypotensive, a statistical outlier, resulting in inadequate treatment, such as that found in this case (Figure 2). It is also common to have falsely low POC glucose meter readings in patients with diabetic ketoacidosis (DKA) and hyperosmolar hyperglycemic nonketotic (HHNK) states.
Why Outliers Are Dangerous.
Reproduced with permission from R. Hellman, MD.
Many POC glucose meters have greater accuracy and precision at only one portion of the glucose measurement range, and if their greatest inaccuracy or imprecision is in the hypoglycemic range, the use of the meter will result in a disproportionate risk for hypoglycemia. A study on 27 glucose meters showed that 41% of the meters did not fulfill even the minimal accuracy requirements under DIN EN ISO 15197 standards [Freckmann et al. Diabetes Technol Ther 2010].
In addition, published data on accuracy and precision of a meter, typically performed in clinical trials by highly trained technicians with new strips, properly stored, with well-cared-for meters, properly calibrated, may not be indicative of results that are obtained in usual patient care. Studies have shown that results are often poorer in less controlled settings [Kristensen et al. Clin Chem 2004; Skeie et al. Clin Chem 2002]. Furthermore, the quality of strip manufacturing may be very variable. A 2008 study showed lot-to-lot variations by several manufacturers, with 5 of 9 instruments showed excessive error in glucose levels due to variation in the hematocrit [Kristensen et al. Diabetes Technol Ther 2008].
Citing this and other examples of the nuanced capabilities of POC meters, Dr. Hellman emphasized that until meter standards improve, the responsibility is on the provider to familiarize itself with the characteristics of each type of meter that its patients and staff may be using. That knowledge must be complemented by patient-specific information (awareness of dietary items/supplements containing interfering compounds, ability of patient to comprehend the meter's instructions, etc.), comprehensive staff training, regularly scheduled quality control, reagent storage (strips are temperature-sensitive), and other pertinent factors.
Given all the potential confounders to POC meter accuracy, it might be argued that they should not be used in hospitals. However, despite the risk of significant error, greater harm has resulted from infrequent or ignored glucose testing, which has led to what Medicare has classified as “never events.” They are medical crises that never should have happened. In 2007, Medicare noted nearly 15,000 such episodes, 76% of which were patients who developed diabetic ketoacidosis after being hospitalized.
To achieve a balance between convenience and clinical utility, Dr. Hellman recommends:
-
The widespread use of POC blood gas multichannel analyzers in critical care
-
FDA requirements for the testing of all POC glucose meters and strips postapproval for accuracy and precision by an independent center; These results should be available to the public
-
All meters should be required to achieve a standard, with a set allowable error (Table 1):
-
Integrated quality assessment programs for POC glucose meters within hospitals and clinics
-
Funding (public or private) for patient education programs, refresher courses, and proficiency testing regarding meter use and maintenance
-
Industry standard interfaces for securely downloading information that is available to the patient and provider without charge
-
Requirements that POC meters that are offered by payers or pharmacy benefit managers be equivalent in accuracy, precision, and ease of learning
-
Studies to determine if meter accuracy standards can be achieved with patients in ordinary, everyday settings
-
Information on the useful life expectancy of the meter to be made available at time of purchase and evaluated by independent nongovernmental agency
-
Patient-oriented information and recommendations for optimal use of each meter system, with online availability
-
Health care providers should be knowledgeable about the meters their patients are using
-
Health care providers should not use POC meters uncritically, especially when the clinical setting makes these values suspect — “trust but verify”
Proposed Standards for Total Allowable Error.
- © 2010 MD Conference Express