Summary
Five key articles that were published during 2009 reflected on ongoing clinical developments in chronic obstructive pulmonary disease (COPD).
- chronic obstructive pulmonary disease
- pulmonary clinical trials
Five key articles that were published during 2009 reflected ongoing clinical developments in chronic obstructive pulmonary disease (COPD), according to a review by Prescott Woodruff, MD, MPH, University of California, San Francisco, CA.
Two of the publications provided details of clinical trial experience with an investigational therapy for COPD. A third article presented data from a recent clinical trial of tiotropium in patients with moderate-to-severe COPD. The final two articles reviewed recent advances in indices for evaluation of COPD clinical status.
Roflumilast is an oral selective phosphodiesterase type 4 (PDE4) inhibitor that is under evaluation as therapy for COPD. The first study was an analysis of two multicenter, placebo-controlled clinical trials that examined the safety and efficacy of roflumilast in patients with moderate or severe COPD (Calverley et al. Lancet 2009). Both trials involved patients aged >40 years with a postbronchodilator forced expiratory volume in one second (FEV1) <50% predicted, bronchitic symptoms, and a history of exacerbations.
Treatment with long-acting anticholinergics or inhaled corticosteroids was not allowed. The primary endpoints were the change in prebrochodilator FEV1 and the rate of moderate (requiring glucocorticosteroid treatment) or severe exacerbations after 52 weeks.
In the two studies combined, 1537 patients received roflumilast (500 μg once daily) and 1554 received placebo. A pooled analysis of the data showed that treatment with roflumilast was associated with a 48-mL improvement in prebronchodilator FEV1 compared with placebo (p<0.0001). The analysis also showed that patients who were treated with roflumilast had 17% fewer moderate or severe exacerbations (p<0.0003). As compared with placebo, roflumilast would prevent one exacerbation for every 3.6 to 5.3 patients who were treated, said Dr. Woodruff.
The adverse event rate was higher in roflumilasttreated patients (67% vs 62% with placebo), with 14% discontinuing treatment due to adverse events versus 11% with placebo. However, most of the difference occurred during the first 12 weeks of treatment. The most common adverse events that were associated with roflumilast were gastrointestinal in nature. Patients who were treated with the PDE-4 inhibitor had weight loss that averaged 2.09 kg, whereas patients who were randomized to placebo gained an average of 0.08 kg during the study.
Another publication reviewed findings from two large multicenter, randomized, placebo-controlled clinical trials of roflumilast as adjunct therapy to long-acting bronchodilators for patients with moderate to severe COPD (Fabbri et al. Lancet 2009). In one trial, patients received roflumilast (500 μg once daily) or placebo in addition to salmeterol for 24 weeks, and in the other, the randomized therapy was added to tiotropium. Concomitant use of inhaled corticosteroids was not allowed.
Both studies involved patients with baseline FEV1 40% to 70% of predicted. In the tiotropium study, eligibility criteria also included cough and positive sputum cytology. The primary endpoint was the change in prebronchodilator FEV1 at 24 weeks. Secondary outcomes included the change in prebronchodilator forced expiratory vital capacity (FVC), change in postbronchodilator FEV1 and FVC, and exacerbation rate.
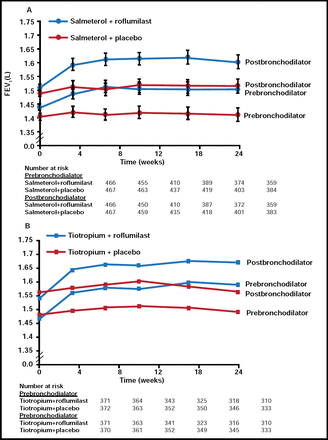
Dr. Woodruff said adjunct therapy with roflumilast was associated with a 49-mL improvement in prebronchodilator FEV1 versus placebo in the salmeterol trial (p<0.0001; Figure 1A) and an 80-mL improvement in the tiotropium trial (p<0.0001; Figure 1B). Both studies demonstrated a consistent reduction in exacerbations with roflumilast. Adverse events with roflumilast were similar to those observed in the other placebo-controlled studies that were described by Dr. Woodruff.
Primary Outcome.
Reprinted from The Lancet, Calverley et al, Volume 374, Issue 9691, 29 August 2009–4 September 2009, Pages 695–703. Copyright 2009 Elsevier.
“Whether the effect of roflumilast would be additive to that seen with inhaled corticosteroids is uncertain,” said Dr. Woodruff. “The differences in roflumilast's effects on outcomes in the tiotropium trial versus the salmeterol study may relate to differences in baseline symptoms, rather than true differences in the additive clinical benefit of roflumilast.”
Because tiotropium is generally reserved for use as adjunct therapy in advanced COPD, few studies have generated data on tiotropium therapy in early-stage COPD, said Dr. Woodruff. A prespecified subgroup analysis of a large randomized, placebo-controlled clinical trial provided additional evidence of tiotropium's safety and efficacy in patients with moderate COPD (Decramer et al. Lancet 2009).
The subgroup analysis focused on 2739 patients with Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage II COPD. The study's primary endpoint was the average rate of decline in pre- and postbronchodilator FEV1. Patients who were treated with tiotropium had an average loss of 43 mL per year compared with 49 mL per year in the placebo group (p=0.024).
Analysis of other outcomes of interest showed that GOLD II patients who were treated with tiotropium had a significant delay in time to first COPD exacerbation compared with placebo (23.1 vs 17.5 months; p<0.0001). Moreover, tiotropium was associated with a mean exacerbation rate of 0.56 per patient-year compared with 0.70 in placebo-treated patients (p<0.0001).
Patients who were treated with tiotropium also had a 26% reduction in the risk of exacerbation that required hospitalization (RR, 0.74; p=0.001) and a 20% reduction in the average number COPD exacerbations that resulted in hospital admission (RR, 0.80; p=0.082).
Dr. Woodruff noted that several clinical indices have been developed to aid clinicians in prognostic assessment of COPD patients. A recently published article examined the performance of two indices—the body mass index, obstruction, dyspnea, exercise capacity (BODE) index and the age, dyspnea, obstruction (ADO) index (Puhan et al. Lancet 2009).
Investigators assessed the accuracy of the BODE index for assessing 3-year mortality in 232 patients with severe COPD and used the same cohort to develop the simplified ADO index (which eliminates the 6-minute walk test that is incorporated into the BODE index). The researchers then validated both indices in an evaluation of 342 patients with moderate to severe COPD.
Evaluation of the two cohorts showed that the BODE index underestimated mortality by 36% in the patients with severe COPD and overestimated mortality by 39% in the cohort of patients with moderate to severe COPD.
The investigators modified the BODE index to incorporate more scoring options for performance on the 6-minute walk test, resulting in an updated index with a 0 to 15 range of possible scores. Application of the updated index to the cohort of patients with moderate to severe COPD resulted in a predicted 3-year mortality of 10.7% compared with a 12% observed mortality.
The ADO index assigns values of 0 to 2 based on FEV1, 0 to 4 for severity of dyspnea, and 0 to 5 for each decade increase in age. Application of the index to the validation cohort yielded a predicted 3-year mortality of 11.8% versus the observed 12%.
Another new index incorporates scores for dyspnea, airflow obstruction, smoking status, and exacerbation frequency (DOSE). British investigators developed the index to provide an assessment tool that can be applied to all COPD patients and health care settings (Jones et al. Am J Respir Crit Care Med 2009). They derived the index from data on 375 patients with COPD who were managed in primary care practices in England. All of the patients had been assessed by the Clinical COPD Questionnaire (CCQ).
Correlational analysis showed that the four components of the DOSE index explained almost half of the variance in health status as determined by the CCQ. Investigators assigned a score of 0 to 3 for each of the four components, and the sum of the four scores provided the total index score. They validated the newly derived index in several cross-sectional and longitudinal samples.
The investigators determined that a DOSE index score >4 was associated with an increased risk of hospital admission and respiratory failure, and the index also correlated with the number of COPD exacerbations in the subsequent year.
“The ADO and the DOSE are more widely applicable than the BODE, because they do not require the 6-minute walk test,” said Dr. Woodruff. “The four components of the DOSE index provide a checklist for specific interventions—smoking cessation, pulmonary rehabilitation, long-acting bronchodilators, and inhaled corticosteroids—for recurrence exacerbations and low lung function.”
- © 2010 MD Conference Express