Summary
There is no evidence of cardiovascular benefit that is associated with long-term treatment with nateglinide and valsartan in patients with impaired glucose tolerance and cardiovascular disease (CVD) or CV risk factors. However, valsartan therapy is associated with a reduction in the incidence of diabetes. This article discusses results from the Nateglinide and Valsartan in Impaired Glucose Tolerance Outcomes Research [NAVIGATOR; NCT00097786] Trial.
- cardiology clinical trials
- diabetes mellitus
There is no evidence of cardiovascular (CV) benefit that is associated with long-term treatment with nateglinide and valsartan in patients with impaired glucose tolerance and cardiovascular disease (CVD) or CV risk factors. However, valsartan therapy is associated with a reduction in the incidence of diabetes. Rury R. Holman, MB, ChB, FRCP, Churchill Hospital, Oxford, United Kingdom, and Robert M. Califf, MD, Duke Translational Medicine Institute, Durham, NC, presented results from the Nateglinide and Valsartan in Impaired Glucose Tolerance Outcomes Research (NAVIGATOR; NCT00097786) Trial.
NAVIGATOR was a double-blind, randomized, multicenter, controlled trial that included 9306 patients with impaired glucose tolerance, defined as fasting plasma glucose (FPG) ≥95 mg/dL and <125 mg/dL and either known CVD if ≥50 years old or ≥1 risk factor for CVD if ≥55 years old. The use of any antidiabetic agent within the last 5 years was an exclusion. Patients were randomized in a 2×2 factorial design to either valsartan (an angiotensin receptor blocker) 160 mg daily or placebo, and to either nateglinide (a short-acting secretagogue) 60 mg 3 times daily or placebo. All study subjects participated in a lifestyle modification program throughout the duration of the study.
One-quarter of participants had known CVD at baseline. The mean age was 64 years, and the median follow-up was 6.5 years for vital status and 5.0 years for incident diabetes. On average, patients in this study were obese at baseline (average BMI 30.5 kg/m2). The three coprimary endpoints for both comparisons of this study were:
-
The incidence of diabetes, defined as fasting plasma glucose (FPG) ≥126 mg/dL (≥7.0 mmol/L) and/or 2-hour plasma glucose ≥200 mg/dL (≥11.1 mmol/L), confirmed by oral glucose tolerance test within 12 weeks;
-
An extended CV composite outcome of CV death, nonfatal myocardial infarction (MI), nonfatal stroke, hospitalization for heart failure, revascularization or unstable angina;
-
The core CV composite outcome of CV death, nonfatal MI, nonfatal stroke, or hospitalization for heart failure.
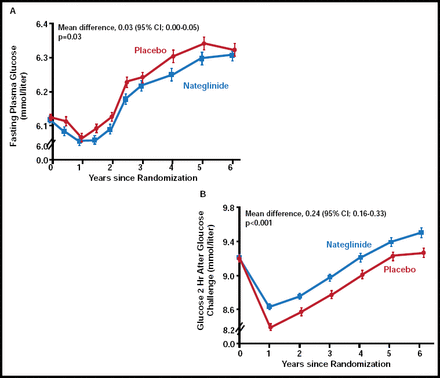
Dr. Holman discussed findings from the nateglinide arm of NAVIGATOR. There was no significant difference between nateglinide (n=4645) and placebo (n=4661) with regard to the extended CV composite outcome or the core CV composite outcome. There was a nonsignificant increase in incident diabetes with nateglinide (36% vs 34%; p=0.05). While patients who were treated with nateglinide demonstrated lower FPG levels over the course of the study (p=0.03), plasma glucose levels 2 hours post-glucose challenge were significantly higher (p<0.001) in the nateglinide group compared with placebo (Figure 1). “These results were unexpected, based on nateglinide's mechanism of action, and the reason for this disparity is unclear,” said Dr. Holman. However, these findings may be due to a decline in drug response over time or an acute withdrawal reaction, as the study drug was withheld on the day of the oral glucose tolerance test.
Changes in Mean Plasma Glucose Levels.
Copyright © 2010 Massachusetts Medical Society. All rights reserved.
Adverse events did not differ between the two treatment groups, with the exception of hypoglycemic events. Nateglinide therapy was associated with an increased risk of hypoglycemia compared with placebo (19.6% vs 11.3%; p<0.001). Of the events in the nateglinide group, 21 were deemed severe, 214 were moderate, and 676 were mild versus 12, 104, and 411 in the placebo group, respectively.
Mean body weight and waist circumference were also higher in the nateglinide group, despite an overall trend in mean body weight reduction throughout the duration of the study. Ten percent of participants lost 5% of their baseline weight by 6 months.
Dr. Califf presented the details of the valsartan (n=4631) versus placebo (n=4675) comparison of NAVIGATOR. He began by pointing out that the difference in the use of concomitant beta-blockers, calcium channel blockers, and diuretics from baseline to last study visit was greater in the placebo arm than in the valsartan arm (p<0.001).
Valsartan did not significantly reduce the incidence of the extended or core CV outcomes. However, there was a 14% relative reduction in the incidence of diabetes in the valsartan group. The cumulative incidence of diabetes was 33.1% in the valsartan group versus 36.8% in the placebo group, amounting to an absolute reduction of 3.8% (HR, 0.86; 95% CI, 0.80 to 0.92; p<0.001). Additional exploratory outcomes of CV death and total mortality were not significantly different.
As with the nateglinide arm of the trial, patients in the treatment arm demonstrated lower FPG over the course of the study. Contrary to the nateglinide results, glucose levels 2 hours post-glucose challenge were also lower in the valsartan treatment arm compared with placebo (p<0.001).
Treatment with valsartan significantly reduced mean sitting blood pressure throughout the duration of the study (p<0.001 for systolic and diastolic measurements). However, hypotension-related adverse events were more common in those who received valsartan (42.4%) than in those who received placebo (35.9%; p<0.001). Other common adverse events were nasopharyngitis, back pain, and arthralgia.
No major safety concerns were identified during the course of the NAVIGATOR study. The rates of major CV events were comparable in all arms of this study, and neither treatment appeared to impact the incidence of CV outcomes. Nateglinide therapy was associated with a higher incidence of hypoglycemia, while valsartan therapy was associated with a higher incidence of hypotension-related adverse events. Valsartan therapy, in combination with lifestyle modification, did reduce the incidence of diabetes in patients with impaired glucose tolerance and CVD or CV risk factors.
Dr. Califf concluded that NAVIGATOR demonstrates that the risks and benefits of therapies can not be predicted accurately based on biology and intermediate measures. Instead, they must be empirically demonstrated with proper randomized clinical trials. While lifestyle modification continues to be the key to diabetes prevention and management, it is important to investigate pharmaceutical options as well, especially in the presence of comorbidities, such as CVD.
- © 2010 MD Conference Express