Summary
Stroke prevention in atrial fibrillation is an evolving story and it is often predicted that the he new oral anticoagulants that are now reaching the market offer numerous advantages over vitamin K antagonists, including oral administration, therapeutic stability that does not require monitoring, once- or twice-daily administration, and a short half-life.
- Arrhythmias
Stroke prevention in atrial fibrillation (AF) is an evolving story, noted Michael D. Ezekowitz, MBchB, DPhil, Thomas Jefferson Medical School, Philadelphia, Pennsylvania, USA, who predicts that the use of vitamin K antagonists (VKAs) in clinical practice will eventually be replaced by newer anticoagulants and that antiplatelet agents will continue to play less of a role in stroke prophylaxis.
The new oral anticoagulants that are now reaching the market offer numerous advantages over VKAs, including oral administration, therapeutic stability that does not require monitoring, once- or twice-daily administration, and a short half-life, said Elaine M. Hylek, MD, Boston University School of Medicine, Boston, Massachusetts, USA.
Dr. Hylek discussed the results of the Stroke Prevention Using the Oral Direct Factor Xa Inhibitor Rivaroxaban Compared with Warfarin in Patients with Nonvalvular Atrial Fibrillation trial (ROCKET AF; see page 12 of this issue). The study involved 14,264 patients who were randomized to either rivaroxaban or warfarin. The patient population had a median age of 73 years, and 44% was aged 75 years or older, which is considerably older than populations who have enrolled in other recent trials of atrial fibrillation (AF). Participants were also higher risk than those of other contemporary trials, with 0%, 13%, and 87% of patients with CHADS2 scores of 0–1, 2, and 3 or more compared with approximately 33% in each group in the RE-LY trial.
The primary endpoint of ROCKET AF was stroke or non-CNS systemic embolism, and the primary safety evaluation was the composite endpoint of major or nonmajor clinically relevant bleeding. The trial was evaluated for noninferiority with a per-protocol (PP) analysis and superiority with an intention-to-treat (ITT) analysis.
The PP analysis demonstrated an event rate of 1.71% per year in the rivaroxaban group compared with 2.16% per year in the warfarin group (HR, 0.79; 95% CI, 0.65 to 0.95; p=0.015), while the ITT analysis showed an annualized event rate of 2.12% with rivaroxaban versus 2.42% with warfarin (p=0.117), demonstrating noninferiority but not superiority [Califf RM et al. AHA 2010].
The relative risk reduction of intracranial bleeding was 33% with rivaroxaban compared with warfarin (p=0.019), although there was a trend toward major bleeding events with rivaroxaban (HR, 1.04; 95% CI, 0.90 to 1.20; p=0.576).
Dr. Ezekowitz focused on dabigatran, recently approved in the United States at the 150-mg and 75-mg daily doses, and apixaban, which is in late-stage clinical trials. Since 80% of dabigatran clearance occurs through the kidneys, dosing depends on kidney function. Twenty-five percent of apixaban is excreted via the kidneys and metabolized through the CYP3A4 pathway. No routine monitoring is required for either drug, and neither affects liver function.
Dabigatran was approved to prevent stroke and systemic embolic events (SEEs) in patients with AF, based on data from the Randomized Evaluation of Long-term anticoagulant therapY noninferiority trial (RE-LY; NCT00262600), in which 18,113 patients with AF and stroke risk were randomized to receive 110 mg or 150 mg dabigatran BID (blinded) or adjusted-dose warfarin (unblinded) [Connolly SJ et al. New Engl J Med 2009].
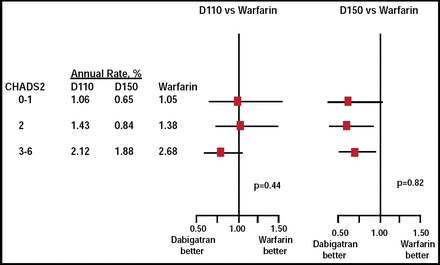
The approval of the 150-mg dose of dabigatran was based on the reduction in stroke/SEEs relative to warfarin. Annual rates of stroke or systemic embolism were 1.69% in the warfarin group versus 1.11% in the 150-mg dabigatran group (RR, 0.65; 95% CI, 0.52 to 0.81; p<0.001 for superiority) [Connolly SJ. N Engl J Med 2010]. The annual rates of major bleeding were 3.63% in the warfarin group versus 3.11% in the 150-mg dabigatran group (p=0.31). Importantly, the relative benefits of dabigatran remained consistent, regardless of whether the patient previously had been treated with a VKA, and were also independent of CHADS2 score (Figure 1).
RE-LY: Stroke and Systemic Embolism in Patients Stratified by CHADS2 Risk Score.
Reproduced with permission from M. Ezekowitz, MBcchB, DPhil.
Apixaban is still under investigation, but results from the Phase 3 Apixaban versus Acetylsalicylic Acid to Prevent Strokes (AVERROES; NCT00496769) trial were presented at the European Society of Cardiology 2010 Congress. This study enrolled 5600 patients with AF who were unsuitable for VKA therapy. Patients were randomized to the study drug or aspirin and followed for 36 months or until study end. The trial was ended early after an interim analysis showed that apixaban reduced the rate of stroke/SEEs by 54% compared with aspirin (1.6 vs 3.6; RR, 0.46; 95% CI, 0.33 to 0.64), with no significantly increased risk in bleeding (RR, 1.14; 95% CI, 0.74 to 1.75; p=0.56) [Connolly SJ et al. ESC 2010].
Although there was a signal of more frequent myocardial infarctions in RE-LY with dabigatran, the modest absolute difference was outweighed by the major benefit that was observed in stroke reduction. Of note, safety during cardioversion also was similar between dabigatran and warfarin, an important clinical observation.
In addition to these and other new anticoagulants (eg, edoxaban, betrixaban), several antiarrhythmic drug options are also under investigation. These include vernakalant, which has a unique ion channel-blocking profile, inhibiting the frequency- and voltage-dependent INa; early activating K+ channel and IKACh, and amiodarone analogs (budiodarone, dronedarone) with improved safety profiles.
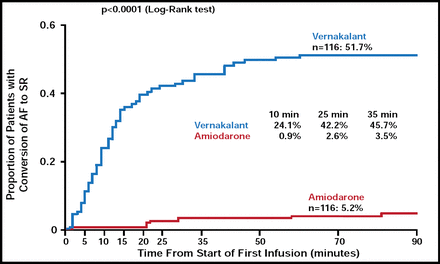
Stefan H. Hohnloser, MD, JW Goethe University, Frankfurt, Germany, discussed the Active-controlled, multicenter, superiority study of Vernakalant injection versus amiodarone in subjects with recent onset atrial fibrillation (AVRO; NCT00668759) trial. More than half of patients who were assigned to intravenous vernakalant (51.7%) reached the primary efficacy point of time to conversion (TTC) from AF to sinus rhythm in 90 minutes compared with just 5.2% of the amiodarone group (p<0.0001), with a median TTC in vernakalant responders of 11 minutes (Figure 2) [Camm AJ et al. JACC In Press].
AVRO: TTC from AF to SR Within 90 Minutes.
Reproduced with permission from S. Hohnloser, MD.
An oral formulation of vernakalant is under development, with a Phase 2b trial demonstrating significant superiority of the 500-mg dose over placebo in patients with AF duration of 3 days to 6 months who had been stratified by their baseline medication. While the median time to relapse in the placebo group was 29 days, it was greater than 90 days in the 500-mg group (p=0.0275, log-rank).
Budiodarone is an amiodarone analog with non-CYP450-mediated metabolism, a lower volume of distribution, and a much shorter half-life than its cousin. The Paroxysmal Atrial fibrillation Study with Continuous Atrial fibrillation Logging (PASCAL) study was the first AF trial to use pacemaker memory to document the recurrence of AF. Patients were randomized to either budiodarone or placebo for 12 weeks. The budiodarone cohorts demonstrated a dose-dependent decrease in AF burden, with an almost 75% reduction in the highest-dose (600 mg BID) group compared with a slight increase in the placebo group (p=0.0056) [Ezekowitz M et al. Heart Rhythm 2009].
Dronedarone, which is already approved in many countries, can be considered a pleiotropic drug, given the variety of its effects. It blocks several ion channels but also has rate-controlling effects, reduces blood pressure, has the capacity for vasodilation, and has antifibrillatory effects in the ventricles as well as the atria [Gautier P et al. J Cardiovasc Pharmacol 2003; Doggrell SA et al. Expert Opin Investig Drugs 2004; Singh BN et al. N Engl J Med 2007]. It is currently being studied in 10,800 patients with permanent AF to assess the effects on major CV events and first unplanned CV hospitalization or death from any cause.
In conclusion, Dr. Ezekowitz noted that “the future looks good for those of us that take care of these patients with respect to the ease of care and the benefits the patients will derive from these innovations.”
- © 2010 MD Conference Express