Summary
Treatment with prescription omega-3 (P-OM3) fatty acids failed to reduce the risk of recurrent atrial fibrillation (AF) after 6 months compared with placebo in patients with paroxysmal AF, according to findings from a large randomized study.
- Cardiology Clinical Trials
- Arrhythmias
Treatment with prescription omega-3 (P-OM3) fatty acids failed to reduce the risk of recurrent atrial fibrillation (AF) after 6 months compared with placebo in patients with paroxysmal AF, according to findings from a large randomized study. P-OM3 was also ineffective in reducing the risk of symptomatic recurrence in patients with persistent AF.
Various doses and preparations of fish oil products have been evaluated to reduce cardiovascular endpoints, with mixed results. The current study examined a high-dose form of pure P-OM3 in patients with AF. Each 1-g capsule of P-OM3 contained approximately 465 mg eicosapentaenoic acid and 375 mg docosahexaenoic acid. Patients were randomized to 8 g daily for the first week, then 4 g daily (n=332) versus placebo (n = 331).
In the prospective, multicenter, double-blind study, 663 patients with paroxysmal (n=542) or persistent (n=121) AF were randomly assigned to treatment with P-OM3 4 g once daily or placebo for 24 weeks. All patients were free from substantial structural heart disease and had normal sinus rhythm at baseline. The primary endpoint was the time to first symptomatic recurrence of AF or atrial flutter in patients with paroxysmal AF. Secondary endpoints included the efficacy and safety of P-OM3 in patients with persistent AF.
Peter R. Kowey, MD, Lankenau Institute for Medical Research, Wynnewood, Pennsylvania, USA, presented the results during a late-breaking clinical trials session.
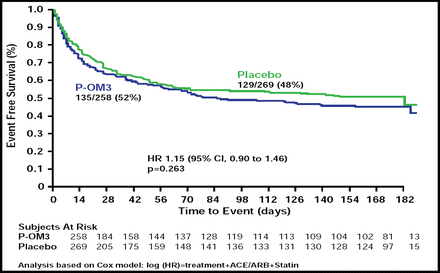
Among patients with paroxysmal AF, P-OM3 failed to reduce the risk of symptomatic recurrence compared with placebo (52% with P-OM3 vs 48% with placebo; HR, 1.15; 95% CI, 0.90 to 1.46; p=0.26; Figure 1). The risk of recurrent AF or atrial flutter was similar in the P-OM3 and placebo groups across patient subgroups, defined by age, gender, race, smoking status, alcohol consumption, angiotensin-converting enzyme inhibitor and angiotensin receptor blocker use, and geographical region.
Time to First Recurrence of Symptomatic AF or Atrial Flutter in Patients with Paroxysmal AF.
Reproduced with permission from P. Kowey, MD.
In an analysis of secondary endpoints, P-OM3 did not reduce the risk of recurrence relative to placebo in patients with persistent AF (50% vs 33%; HR, 1.64; 95% CI, 0.92 to 2.92; p=0.09) or in the combined study group of paroxysmal and persistent AF (52% vs 46%; HR, 1.22; 95% CI, 0.98 to 1.52; p=0.08). P-OM3 also failed to reduce the annualized number of rescue episodes versus placebo (4.2 vs 2.4; p=0.07), the cumulative frequency of symptomatic AF or atrial flutter episodes (8.3 vs 6.8; p=0.32), or symptomatic AF recurrences (8.3 vs 6.9; p=0.24).
Although P-OM3 did not provide a clinical benefit, fish oil treatment did result in several favorable biological changes. Compared with the placebo group, the P-OM3 group had a lower ventricular rate during the first AF recurrence. Patients in the P-OM3 group also had decreased triglyceride and very low-density lipoprotein levels at Week 24, lower systolic blood pressure levels at Week 24, and increased plasma levels of the omega-3 fish oils eicosapentaenoic acid and docosahexaenoic acid at Week 4 and Week 24.
P-OM3 was well tolerated. Adverse events occurred with similar frequency in the P-OM3 and placebo groups, including nausea (5% vs 4%), dizziness (4% vs 3%), urinary tract infection (4% vs 4%), sinusitis (3% vs 4%), and peripheral edema (4% vs 2%).
In summary, findings from this large prospective trial do not support the use of P-OM3 to reduce the risk of recurrent AF, investigators said. However, the results do not exclude the potential for a benefit with high-dose P-OM3 therapy in combination with other antiarrhythmic drugs or in different patient populations, such as high-risk primary prevention patients or in postoperative AF. Future prospective trials may examine the role of P-OM3 in these clinical settings.
Results of this study were published simultaneously in the Journal of the American Medical Association. Kowel P et al. JAMA 2010.
- © 2010 MD Conference Express