Summary
This article discusses the results of the first human randomized controlled trial of sympathetic renal denervation as a treatment for treatment-resistant hypertension, which showed substantial reductions in systolic blood pressure.
- Renal Disease
- Hypertension & Kidney Disease
- Hypertensive Disease
- Interventional Radiology Clinical Trials
The role of the sympathetic nervous system in hypertension has been known for some time, but its potential as a therapeutic avenue has been overshadowed by drug therapy. Murray D. Esler, MD, Baker IDI Heart and Diabetes Institute, Melbourne, Australia, presented the results of the first human randomized controlled trial of sympathetic renal denervation as a treatment for treatment-resistant hypertension, which showed substantial reductions in systolic blood pressure (SBP).
The International, Multicenter, Prospective, Randomized, Controlled Trial of Endovascular Selective Renal Sympathetic Denervation for the Treatment of Hypertension (Symplicity HTN-2; NCT00888433) was conducted in 24 centers in Europe, Australia, and New Zealand. The primary study endpoint was change in office-based automated SBP between baseline and 6 months. Secondary endpoints included acute and chronic procedural safety issues and home/ambulatory BP reductions.
Patients aged between 18 and 85 years with an office SBP ≥160 mm Hg (≥150 mm Hg in individuals with type 2 diabetes) and a bilateral single main renal artery >20 mm long and 4 mm in diameter who were taking at least 3 antihypertensive medications were eligible for this trial. Patients with renal artery duplication or stenosis, eGFR <45 mL/min, type 1 diabetes, or unstable angina/recent cerebrovascular accidents were excluded.
A total of 190 patients were screened, and 106 were randomly assigned to renal denervation (RDN) plus continuing medical therapy (n=52) or continuing medical therapy only (n=54). Interestingly, 19% of the screened failures were patients who did not meet the SBP threshold after 2 weeks of documented medication compliance. Another 16% of screened failures were due to ineligible anatomy (eg, multiple renal arteries). Study participants had a mean age of 58 years, mean baseline SPB of 178 mm Hg, and average BMI 31 kg/m2 and were taking 5.3 antihypertensive medications on average. More than 75% of study participants had been taking antihypertensive medications for more than 5 years. Almost all participants were taking angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBs). Other drug classes included calcium channel blockers (79% of RDN patients vs 83% of control patients), beta-blockers (83% vs 69%), and diuretics (89% vs 91%). Mean eGFR was 77 ml/min/1.73 m2 in the denervation group versus 86 ml/min/1.73 m2 in the control group (p=0.013)
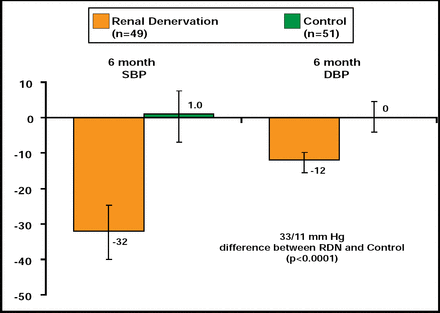
Bilateral RDN was performed successfully in all 52 patients who were assigned to this study arm. At 6 months, patients in the RDN-treated group demonstrated significant decreases in SBP and DBP (33/11 mm Hg; p<0.0001) relative to control (Figure 1), with 84% of patients who were undergoing RDN experiencing a ≥10-mm Hg decrease in SBP (vs 35% of controls; p<0.0001). Similar decreases were noted for home and 24-hour ambulatory BP. BP reductions were progressive over the observation period, indicating the possibility of greater benefits over time. Use of antihypertensive medications declined in 20% of patients who were assigned to RDN versus 6% of control patients (p=0.04).
Primary Endpoint.
Reproduced with permission from M. Esler, MD.
There were no serious device- or procedure-related complications. No acute renal artery damage or radiofrequency dosing-related abnormalities were detected in the 6-month follow-up period.
This treatment appears promising for hypertensive patients whose blood pressure does not achieve the treatment goal using currently available therapies and affirms the crucial relevance of renal nerves in the persistence of elevated BP. “This procedure provides a revolutionary, nondrug method for controlling high blood pressure in patients who are unresponsive to multiple antihypertensive drugs,” Dr. Esler said.
A randomized US-based trial is in development, while the future application of the technique in patients with less severe essential hypertension is also under consideration.
This article was published simultaneously in The Lancet. Symplicity HTN-2 Investigators. Lancet 2010.
- © 2010 MD Conference Express