Summary
This article discusses results from the Determining the Efficacy and Tolerability of CETP Inhibition with Anacetrapib [DEFINE; NCT00685776] trial, which showed that anacetrapib safely and substantially lowered LDL-C and raised HDL-C levels in patients with coronary heart disease.
- Cardiology Clinical Trials
- Lipid Disorders
Cholesteryl ester transfer protein (CETP) inhibition can raise high-density lipoproteins (HDLs) and, in some cases, lower low-density lipoproteins (LDLs). Development of the first CETP agent to be evaluated in a Phase 3 clinical trial—torcetrapib—was stopped, however, when it was found to be associated with increased mortality and cardiovascular events [Barter PJ et al. N Engl J Med 2007]. Christopher P. Cannon, MD, Brigham and Women's Hospital, Boston, Massachusetts, USA, reported results from the Determining the Efficacy and Tolerability of CETP Inhibition with Anacetrapib (DEFINE; NCT00685776) trial, which showed that anacetrapib safely and substantially lowered LDL-C and raised HDL-C levels in patients with coronary heart disease (CHD).
DEFINE was a Phase 2, randomized, double-blind, placebo-controlled trial to assess the efficacy and safety of anacetrapib in patients with CHD or CHD risk equivalents (Framingham Risk Score >20%). In addition to the CHD requirement, participants were required to be aged between 18 and 80 years and have LDL-C ≥50 but ≤100 mg/dL, HDL-C <60 mg/dL, and triglycerides (TG) ≤400 mg/dL.
The primary study endpoints were the percentage change from baseline in LDL-C after 24 weeks of treatment and the safety and side effect assessments through 76 weeks. Key efficacy endpoints included HDL-C, apoB, apoA1, non-HDL-C, and TG levels at Weeks 24 and 76 and LDL-C at Week 76. Subjects (mean age 63 years, 78% men) were randomly assigned to receive 100 mg anacetrapib (n=811) or placebo (n=812) once daily for 18 months, followed by a 3-month poststudy follow-up.
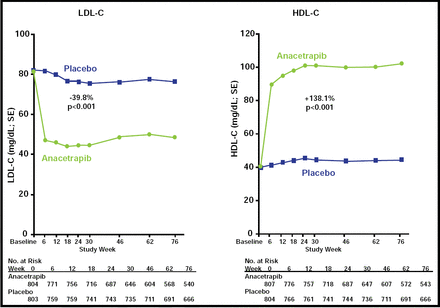
At Week 6, LDL-C levels decreased by 40% (p<0.001) and HDL-C levels increased by 138% (p<0.001) compared with placebo (Figure 1). Effects on other lipid parameters are shown in Table 1. Anacetrapib did not exhibit the adverse cardiovascular effects that have been seen with torcetrapib, including changes in blood pressure, electrolyte disturbances, and elevations in aldosterone levels. Cardiovascular events occurred in 16 patients in the anacetrapib group (2.0%) and 21 patients who received placebo (2.6%; p=0.40). Using Bayesian analysis, the investigators determined that this event distribution indicated a 94% predictive probability that anacetrapib would not be associated with the 25% increase in cardiovascular adverse events that was seen with torcetrapib. Furthermore, the composite of all-cause death, MI, unstable angina, stroke, or revascularization was lower in the anacetrapib group (3.3%) compared with the placebo group (5.3%; p=0.048). This was mostly attributed to a lower rate of revascularization with anacetrapib (1.0% vs 3.5%; p<0.001).
Other Lipid Parameters.
Effects on LDL-C and HDL-C.
Copyright © 2010 Massachusetts Medical Society. All rights reserved.
“This drug has profound effects on HDL going to new highs and with LDL going to additional lows,” Dr. Cannon remarked in an interview. Additional, larger studies, soon to be initiated, are needed to establish the clinical benefit of anacetrapib, expand the ethnic diversity of the study population, and provide more insight into the long-term safety of reducing LDL-C to extremely low levels.
Thomas F. Lüscher, MD, University of Zurich, Zurich, Switzerland, discussed the design and results of the DEFINE trial. He concluded the trial was well designed and that CETP inhibition with anacetrapib resulted in impressive changes in lipid profile beyond those that were achieved with statins without increasing blood pressure.
He further stated, however, “It remains to be shown that the HDL particles during treatment with anacetrapib are biologically normal.”
This article was published simultaneously in The New England Journal of Medicine. Cannon CP et al. N Engl J Med 2010.
- © 2010 MD Conference Express