Summary
Rivaroxaban was noninferior to warfarin in preventing all-cause stroke and noncentral nervous system embolism in patients with atrial fibrillation, according to new findings from the Rivaroxaban Once-Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation [ROCKET-AF].
- Arrhythmias
- Cerebrovascular Disease Clinical Trials
- Cerebrovascular Disease
Rivaroxaban was noninferior to warfarin in preventing all-cause stroke and noncentral nervous system (CNS) embolism in patients with atrial fibrillation (AF), according to new findings from the Rivaroxaban Once-Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET-AF).
Rivaroxaban, an investigational factor Xa inhibitor, has been shown to be an effective option for oral anticoagulation in patients who are at risk for venous thromboembolism. ROCKET-AF was designed to compare rivaroxaban with warfarin, the current standard of care for patients with AF who are at risk of stroke and systemic embolism. Kenneth Mahaffey, MD, Duke Clinical Research Institute, Durham, North Carolina, USA, presented results from ROCKET-AF.
In ROCKET-AF, 14,236 patients with AF and a history of stroke or multiple cardiovascular risk factors were randomly assigned to treatment with rivaroxaban 20 mg daily (n=7111) or dose-adjusted warfarin that was titrated to an international normalized ratio (INR) of 2.0–3.0 (n=7125).
Most patients in ROCKET-AF (86%) had a CHADS2 score of 3 or higher, which is substantially higher than the risk profile of patients who have been enrolled in other major stroke prevention trials. Patients in the warfarin group spent only 57.8% of time in the therapeutic range (TTR), reflecting the challenges of vitamin K antagonist-based anticoagulation therapy as well as the high-risk study population. In comparison, the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial of dabigatran versus warfarin in AF reported a TTR of 64% [Connolly S et al. New Engl J Med 2009].
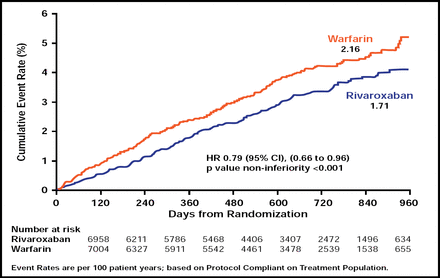
In the primary efficacy analysis, rivaroxaban met the criteria for noninferiority compared with warfarin (p<0.001). The cumulative event rate for stroke and non-CNS embolism was 1.71 per 100 patient-years in the rivaroxaban group and 2.16 in the warfarin group (HR, 0.79; 95% CI, 0.66 to 0.96; Figure 1).
Prevention of Stroke or Non-CNS Embolism With Rivaroxaban Versus Warfarin in Patients with AF.
Reproduced with permission from K. Mahaffey, MD.
Rivaroxaban did not meet the criteria for superiority in reducing stroke and non-CNS embolism compared with warfarin in patients in the intent-to-treat (ITT) analysis that also included noncompliant patients (2.12 vs 2.42; p=0.117). Among patients who remained on treatment during the trial, rivaroxaban did significantly reduce the primary endpoint (1.70 vs 2.15; p=0.015).
In the per-protocol analysis of event rates, rivaroxaban also reduced the combined endpoint of vascular death, stroke, and embolism compared with warfarin (3.11 vs 3.63; p=0.034), as well as the individual endpoints of hemorrhagic stroke (0.26 vs 0.44; p=0.024) and non-CNS embolism (0.04 vs 0.19; p=0.003). There was also a trend toward reduced all-cause mortality in the rivaroxaban group compared with placebo (1.87 vs 2.21; p=0.073). In an ITT analysis, however, rivaroxaban remained superior to placebo only with regard to hemorrhagic stroke reduction (0.26 vs 0.44; p=0.012). Rivaroxaban had no effect on ischemic stroke relative to warfarin in the on-treatment (1.34 vs 1.42; p=0.581) or ITT analysis (1.62 vs 1.64; p=0.916).
Patients in the rivaroxaban and warfarin groups had similar overall rates of major and nonmajor clinically relevant bleeding (14.9 vs 14.5; p=0.44). However, rivaroxaban significantly reduced the risk of intracranial bleeding, with 55 events in the rivaroxaban group and 84 events in the warfarin group (p=0.019). Moreover, although the overall event rate for major bleeding was similar in the rivaroxaban and the warfarin groups (3.60 vs 3.45; p=0.58), rivaroxaban significantly reduced the risk of death that was caused by major bleeding (0.24 vs 0.48; p=0.003). Conversely, rivaroxaban increased the risk of transfusion (1.65 vs 1.32; p=0.044) and hemoglobin reduction ≥2 g/dL (2.77 vs 2.26; p=0.019).
Patients reported adverse events with similar frequency in the rivaroxaban and warfarin groups, including any serious adverse event (37.3% vs 38.2%) and any adverse event that led to study drug discontinuation (15.7% vs 15.2%).
Rivaroxaban joins dabigatran as another potential alternative for standard anticoagulation with warfarin therapy in patients who are at risk for stroke, investigators said. Rivaroxaban was given as a once-daily agent in this trial and is a factor Xa inhibitor, while dabigatran is a direct thrombin inhibitor that was given twice daily in RE-LY. Dabigatran 150 mg BID (75 mg BID for severe renal impairment) was recently approved by the US Food and Drug Administration for the prevention of stroke and systemic embolism in patients with AF. Rivaroxaban has been approved for use in DVT/PE and is currently under review for use in AF.
- © 2010 MD Conference Express