Summary
This article discusses the epidemiology of subarachnoid hemorrhage (SAH) and provided insight into patient outcomes, therapies, and treatments.
- ischemia
- interventional techniques & devices
Mathew Flaherty, MD, University of Cincinnati, Cincinnati, OH, discussed the epidemiology of subarachnoid hemorrhage (SAH) and provided insight into patient outcomes. In most Western populations, the incidence of SAH is ∼6- to 10 cases/1,000,000 persons. In the United States, African- and Mexican-Americans have double the risk of SAH compared with Caucasians. The incidence in women averages 1.24 (range 1.09 to 1.42)-times higher than in men. This gender difference begins at age 55 years and increases thereafter [de Rooij NK et al. J Neurol Neurosurg Psychiatry 2007]. Smoking and hypertension are the most important risk factors for SAH [Feigin VL et al. Stroke 2005]. Despite only accounting for 5% of all strokes, SAH remains an important subtype, because it occurs in younger patients, carries a significant morbidity and mortality risk, and incurs higher costs compared with other subtypes.
Poor clinical status on admission is the most significant indicator of a poor outcome, followed by rebleeding. Overall, 10% to 15% of patients with an SAH die before hospitalization, with a 30-day case fatality of 20% to 45%. Lingering problems that are common among survivors at 1 year include memory, mood, and speech disturbances and difficulty with self-care [Hackett ML et al. Neurology 2000].
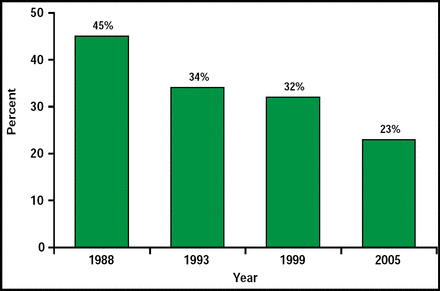
In concluding, Dr. Flaherty noted that things are not all doom and gloom. SAH case fatality is declining (Figure 1).
Decline in SAH Fatalities in Cincinnati.
Reproduced with permission from M. Flaherty, MD.
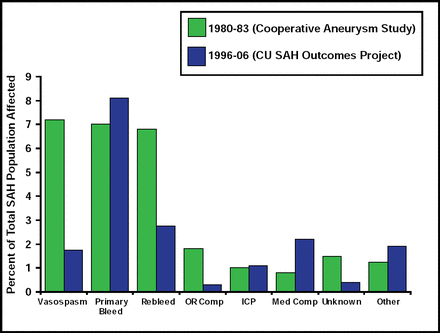
Treatment of SAH patients in the ICU typically involves preventing rebleeding and minimizing delayed ischemia from vasospasm. This approach was based on data from the International Cooperative Study on the Timing of Aneurysm Surgery, which found that vasospasm was the primary cause of death (7%) among SAH patients [Kassel NF et al. J Neurosurg 1990]. Much progress has been made in preventing vasospasm, however, such that today, that number is closer to 1%. Currently, the primary cause of death in these patients is the acute effects of a primary severe bleed (Figure 2). Ventriculostomy is often the only tool for acute brain protection in poor-grade SAH patients.
Causes of Hospital Death After SAH.
Reproduced with permission from S. Mayer, MD.
Global cerebral edema develops in 20% of SAH patients and is an independent risk factor for mortality, cognitive dysfunction, and poor outcome after SAH [Claassen J et al; Kreiter KT et al. Stroke 2002]. Infusion of 23.5% hypertonic saline 2 mL/kg has been shown to be an effective treatment for brain swelling, in that it has been shown to decrease intracranial pressure (74%), decrease cerebral perfusion pressure (27%), and increase cerebral blood flow (23%) in a small group of 10 patients. Peak effect occurred between 20 and 60 minutes of infusion [Tseng M-Y et al. Stroke 2003].
The protocol for SAH at Dr. Mayer's institution is intravascular (IV) loading with epsilon aminocaproic acid (Amicar) upon diagnosis (4-g IV load and 1 g every hour until 72 hours or aneurysm repair). This protocol has been shown to decrease rebleeding in treated patients by 2.7% versus nontreated patients (11.4%). In the future, Dr. Mayer sees the use of more advanced monitoring of brain tissue oxygen and transplumonary thermodilution, which can indicate global end-diastolic volume.
The issue of whether an endovascular (coil) or microsurgical (clip) approach is a better way to treat aneurysmal SAH remains debatable, said B. Gregory Thompson, MD, University of Michigan, Ann Arbor, MI. The prevailing view is that interventional techniques are less invasive and less morbid at initial treatment. The superiority of coiling over clipping was shown in the International Subarachnoid Aneurysm Trial (ISAT), which reported an absolute risk reduction of 6.9% after 1 year with the use of coils compared with patients who received endovascular (clipping) treatment [Molyneux A et al. Lancet 2002]. Dr. Thompson argued that ISAT had methodological problems because of the subjective nature of the eligibility definition. Selection bias may have been compromised because of the use of clinical equipoise, which excluded 77% of patients. Documented experience of the surgeons was not required; thus, it was difficult to determine the equivalency of the technical expertise of the endovascular and surgical practitioners.
External validity was not shown when the results of the ISAT were compared with other similar trials [Harbaugh RE. Lancet 2003]. The surgical morbidity appeared to be relatively high, particularly considering the clinical status of the patients at the time of randomization and the size of the aneurysms. The ISAT patient cohort, compared with other randomized, prospective aneurysm studies, had lower average Hunt and Hess (HH) scores, smaller aneurysm sizes, and a lower percentage of procedures in the posterior circulation location, a more difficult area to treat.
After evaluating outcomes in other trials, Dr. Thompson cited specific factors that favored endovascular or microsurgical treatment. Factors that favor microsurgical treatment include: SAH HH grade between 1 and 3; an anterior circulation location (PICA, SCA, supraclinoid ICA, MCA); <3 mm in size or giant aneurysm with neck >5 mm; a saccular, fusiform, or abnormal branch morphology; younger patients (<60 years of age); and hematoma (mass effect or edema).
Factors that favor endovascular treatment include: all SAH HH grades; posterior circulation (basilar apex, paraclinoid ICA, petrocarvernous); small to medium in size with a narrow neck (<5 mm); morphology may include saccular and calcified aneurysms; >60 years of age; medical comorbidities; and no hematoma
Giuseppe Lanzino, MD, Mayo Clinic, Rochester, MN, followed Dr. Thompson and took the opposite point of view, arguing that the ISAT was a well-designed study that supported the use of coils and that coiling was superior to clipping, citing evidence from the Kuopie and BRAT studies.
The Kuopio study reported comparable outcomes at one year after early surgical and endovascular treatment of ruptured intracranial aneurysms. Though superficial brain retraction deficits (p<0.001) and ischemic lesions in the territory of the ruptured aneurysm (p=0.025) were more frequent in the surgical group, 39-month Kaplan-Meier analysis revealed equal survival in both treatment groups [Koivisto T et al. Stroke 2000].
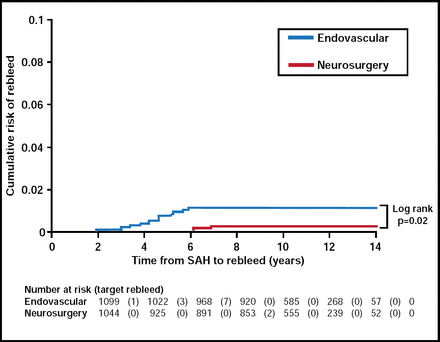
Dr. Lanzino argued that the ISAT is notable, in that follow-up data at one year were available in a high percentage (98%) of eligible patients and that it was the only study of this nature to use patient self-assessment to score outcome. In the ISAT, coil embolization appeared to be safer than clip ligation at one year, while clip occlusion had better long-term efficacy at preventing rebleeding. Rebleeding rates were very low for both groups (Figure 2), while endovascular patients had lower mortality rates compared with patients who received a clip (11% vs 14%; p<0.05).
Cumulative Risk of Rebleed.
Reproduced with permission from G. Lanzino, MD.
Age affected outcome, in that better long-term protection from SAH that is afforded by clip placement may give this treatment an advantage in life expectancy for patients aged < 40 years [Mitchell P et al. J Neurosurg 2008]. Early evidence from the BRAT and other studies mirrored the results from the ISAT [van der Schaaf I et al. Cochrane Database Syst Rev 2005].
Dr. Lanzino concluded that if a ruptured aneurysm is considered suitable for endovascular or surgical treatment, there is firm evidence that coiling is associated with better outcomes, while younger patients (<40 years) may benefit from surgery. From his own clinical experience, he believes that the durability of endovascular procedure is steadily improving.
- © 2010 MD Conference Express