Summary
Although advances seemed elusive throughout past years, immunotherapy is beginning to fulfill its promise in the treatment of non—small cell lung cancer (NSCLC). For years, researchers have been intrigued by the notion of trying to enlist the immune system to attack tumors; however, little was actually realized until the mechanisms of immune evasion used by tumor cells were defined and immune regulators of the programmed death and programmed death ligand (PD-1—PD-L1) pathway were recently devised. This article discusses the advent of immunotherapy that was made possible by the development of immune checkpoint inhibitors that target the PD-1—PD-L1 pathway.
- Cancer
- Respiratory Cancers
- Oncology
- Pulmonary & Respiratory Medicine
Although advances seemed elusive throughout past years, immunotherapy is beginning to fulfill its promise in the treatment of non-small cell lung cancer (NSCLC). For years, researchers have been intrigued by the notion of trying to enlist the immune system to attack tumors; however, little was actually realized until the mechanisms of immune evasion used by tumor cells were defined and immune regulators of the programmed death and programmed death ligand (PD-1-PD-L1) pathway were recently devised.
In the keynote lecture given on March 26,2014, at the 4th European Lung Cancer Conference (ELCC), Julie Brahmer, MD, of the Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University, Baltimore, Maryland, USA, described the advent of immunotherapy that was made possible by the development of immune checkpoint inhibitors that target the PD-1–PD-L1 pathway.
Dr. Brahmer began the immunotherapy story with a brief overview of the pathway these new antibodies target. She then presented Phase 1 trial results for 2 of the more promising agents with activity in NSCLC, noting that data from Phase 2 and 3 trials were not yet mature for these quite recently developed antibodies.
CHECKPOINT INHIBITION OF T-CELL ACTIVATION
These checkpoint inhibitors have activity in the signaling pathway of T-cell activation that is mediated in part by programmed death PD-1 receptor, a molecule found on antigen presenting and tumor cells that initiates signaling to the T-cell via the PD-L1 leading to T-cell energy and immune suppression. PD1-PD-L1 is just one of several redundant pathways in this signaling cascade, Dr. Brahmer pointed out.
According to Dr. Brahmer, lung cancer tumor cells achieve immune evasion by several mechanisms, including defective antigen presentation, upregulation or secretion of immunosuppressive cytokines, and deregulation of checkpoint pathways.
Checkpoint inhibitors act at different points in the PD-1-PD-L1 pathway, but their function is to inhibit the interaction of this pathway between T-cells and cancer cells. A striking feature of the response with any of these agents is the durability of the response, which persists even when patients go off therapy, suggesting some sort of immune memory has been created, said Dr. Brahmer.
BENEFIT FROM PD-L1 AGENTS SIMILAR TO THAT OF CHEMOTHERAPY
Two recently developed PD-L1 agents have shown results that, according to Dr. Brahmer, are comparable to those seen with chemotherapy in patients with NSCLC but are not as dramatic as the results seen with some targeted therapies in patients with the appropriate genetic profile. BMS-936559 and MPDL-3280A are both immunoglobulin G (IgG) monoclonal antibodies targeting PD-L1 and preventing the PD-1-PD-L1 complex formation, thereby stopping the signaling cascade and restoring the immune response.
In a recent Phase 1 trial of BMS-936559, a response rate of 10% was demonstrated in 49 patients with NSCLC; stable disease (SD) and progression-free survival (PFS) at 24 weeks of 12% and 31%, respectively, were demonstrated [Brahmer J et al. New Engl J Med 2012]. A trial of MPDL-320A in 53 patients demonstrated a response rate of 23%; at 24 weeks post treatment initiation, SD was achieved by 17% of patients and the PFS rate was 45% [Soria JC et al. ECC 2013 Abstract 3408].
The response to MPDL-3280A was higher in patients who were smokers. The overall response rate (ORR) in the cohort of 53 patients who achieved partial response (PR) was 26% for patients who were current or former smokers versus 10% who were never smokers [Soria JC et al. ECC 2013 Abstract 3408]. Dr. Brahmer explained that the current thought is that smoking is associated with a higher rate of mutations that could be associated with an increased immune recognition or response. Restoring the immune surveillance mechanisms should, therefore, show greater activity in cells with the most DNA damage.
An impressive feature of these immune checkpoint inhibitors is the prolonged duration of response seen in the patients who benefit from immunotherapy; the responses are durable, even when the drug is stopped, and were observed in patients who received just one dose. To Dr. Brahmer, this suggests that the immune system is being “reset” by these agents, resulting in continued activity long after the drug is stopped.
AGENTS TARGETING THE PD-1 RECEPTOR
A second route of modulating this checkpoint pathway is with investigational drugs that target the PD-1 receptor; nivolumab and MK-3475 are also monoclonal IgG antibodies, but these are specific to PD-1. One trial of nivolumab given as a single agent showed an ORR of 17.1%, but higher response rates of 24.3% were seen at the optimized dose (3 mg/kg twice a day) [Spigel D. ASCO 2013]. Again, the duration of response was impressive, and the estimated median duration of response (DOR) was 74.0, at all dose levels; median DOR was 63.9, 74.0, and 83.1 weeks at doses of 1,3, and 10 mg/kg, respectively. Most of the patients who respond benefit for a long period of time without signs of disease progression, according to Dr. Brahmer. She pointed out that 2 patients in her clinic who had progressed on several prior therapies and had an expected survival of 4 to 6 months are still alive 3 years after receiving nivolumab in a clinical trial.
Toxicities differed with each agent and especially between drugs that block PD-1 and those targeting PD-L1 (Table 1).
PD-1 Checkpoint Inhibition: Toxicities
Just as with targeted therapy, a remaining challenge is identifying just which patients are likely to benefit from checkpoint-modulating agents. “We have yet to find a way of identifying those patients, other than to try them on the drug to see if they respond,” Dr. Brahmer said. Response has been found to be associated with the level of PD-Llexpression [Topolian S et al. ASCO 2013; Gosso 1 et al. ASCO 2013; Herst Ret al. ASCO 2013], making it a plausible candidate marker for treatment, but other markers need to be developed. The question remains whether PD-L1 positivity is predictive of response or prognostic.
EPIGENETIC THERAPY PLUS IMMUNE MODULATION IN NSCLC
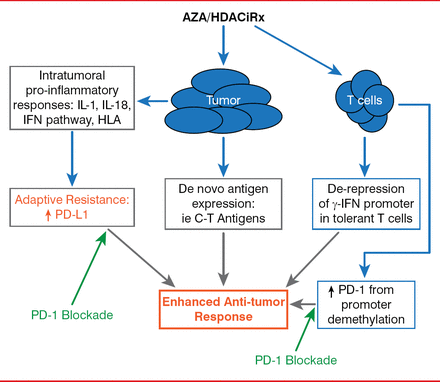
Finally, Dr. Brahmer explored the possibility of combination therapies and whether there could be a synergistic effect between immune checkpoint inhibitors, agents that target other pathways, and co-stimulatory pathways, or with epigenetic therapy.
Synergy Between Epigenetic Modulation and PD-1 Pathway Blockade
Reproduced with permission from 1 Brahmer, MD.
Dr. Brahmer outlined the strategy behind an ongoing trial of epigenetic therapy followed by immune modulation of a checkpoint pathway that is currently underway in the lab and in the clinic. In this study, combination epigenetic therapy consists of “priming” the patient by first targeting epigenetic alterations that drive oncogenesis within the tumor using a combination of the demethylating agent azacitidine and the histone deacetylase inhibitor entinostat. Epigenetic therapy is immediately followed by immunotherapy with nivolumab, thus unleashing a “perfect storm” of antitumor therapy. Preliminary results are anticipated by the end of 2014.
The future of NSCLC therapy will be broadened by immunotherapy, especially checkpoint modulating agents, according to Dr. Brahmer, but this new era of treatment may be dependent on finding the mechanism of immune evasion in each patient to individualize treatment.
- © 2014 MD Conference Express®