Summary
Particular sections of the much anticipated 2013 American College of Cardiology (ACC)/American Heart Association (AHA) cardiovascular (CV) prevention guidelines have received both applause and criticism. This article presents experts' views on a few of the more divisive recommendations.
- Coronary Artery Disease
- Lipid Disorders
- Cardiology Guidelines
- Coronary Artery Disease
- Lipid Disorders
- Cardiology Guidelines
- Cardiology
Particular sections of the much anticipated 2013 American College of Cardiology (ACC)/American Heart Association (AHA) cardiovascular (CV) prevention guidelines have received both applause and criticism. In this session, several experts presented their views on a few of the more divisive recommendations.
One controversial recommendation in both primary and secondary prevention in the 2013 Cholesterol Guidelines [Stone NJ et al. J Am Coll Cardiol 2013; Circulation 2013] is the shift away from titrating drug therapy to specific low-density lipoprotein-cholesterol (LDL-C) targets as previously advocated. This change by the authors was based on review of the applicable clinical trials. For example, trials that have consistently demonstrated the efficacy and safety of statins in multiple patient populations have not tested strategies of titration to LDL (or other lipid marker) targets. These trials tested strategies of fixed-dose statin versus placebo, and later fixed-dose intensive versus standard statin therapy. In each comparison, the former demonstrated greater reductions in CV outcomes such as myocardial infarction, stroke, and CV death with minimal safety or tolerability concerns. Karol E. Watson, MD, PhD, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, California, USA, believes recommendations for a fixed-dose strategy are an improvement as the recommended treatment strategy is now tied directly to scientific evidence.
Dr. Watson also agreed with the recommendation of medications proven to confer the greatest CV risk reduction. Consistent with the evidence, the guideline authors reviewed the available cholesterol therapies and found that statins resulted in risk reduction of major coronary events, coronary revascularization, stroke, and other major vascular events. In line with the evidence, statins should form the foundation of CV risk reduction by pharmacologic lipid-modifying therapy (Table 1) [Kearney PM et al. Lancet 2008]. In fact, data supporting a clinical benefit for other lipid-modifying drugs is minimal.
Statin Effects on Major Vascular Events
While the new guidelines recommend statin therapy in patients with a prior atherosclerotic event or diabetes, for those between the ages of 40 and 75 years with an LDL between 70 and 189 mg/dL, they recommend use of a risk estimator to assess whether statin therapy would provide net benefit. For patients agedn <40 and >75 years without a history of diabetes or prior atherosclerotic event, the authors do not provide specific guidance as data in these groups are limited. In regards to the risk estimator, Dr. Watson applauded the guideline authors for expanding the risk estimator outcome to coronary heart disease (CHD) death, myocardial infarction (MI), and stroke (not just CHD and MI). The risk estimator now also considers gender, age, race, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure (BP), whether the patient is receiving BP treatment, and their diabetes and smoking status. It also provides information from ages 20 to 59 years on lifetime CV risk. Finally, the guideline authors encourage discussion between the clinician and patient regarding the potential risk reduction benefits and adverse effects of any therapy, drug-drug interactions, incorporation of other relevant information (eg, family history, coronary artery calcium [CAC] score), and patient preference.
James S. Forrester, MD, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, California, USA, believes that replacing the LDL target strategy with one based on fixed-dose statins is unwise as other lines of evidence (eg, genetic data) support a “lower is better” approach to LDL management. Instead, Dr. Forrester recommends a hybrid of fixed statin dose and LDL target strategy as the best approach for prevention.
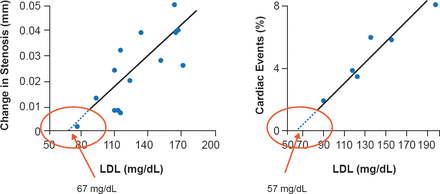
Dr. Forrester discussed two clinical studies that support increased efficacy with further LDL reduction. One suggested that the progression of atherosclerosis and the incidence of CHD events are minimized when LDL is lowered to <70 mg/dL (Figure 1) [O'Keefe JH, Jr. et al. J Am Coll Cardiol 2004]. The other, from the Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin study [JUPITER], indicated that participants attaining LDL-C <50 mg/dL had an additional 21% absolute risk reduction in CV events rates compared with the entire study population. Decreases in LDL beyond prior targets also have not been consistently associated with safety or tolerability concerns [Hsia J et al. J Am Coll Cardiol 2011].
Optimal LDL-C Level to Minimize Atherosclerosis Progression and Coronary Heart Events
LDL=low-density lipoprotein.
Reproduced from O'Keefe JH, Jr et al. Optimal low-density lipoprotein is 50 to 70 mg/dl: lower is better and physiologically normal. J Am Coll Cardiol 2004; 43(11):2142–2146. With permisison from Elsevier.
Dr. Forrester's second criticism is regarding the risk estimator in which age may be overemphasized, potentially resulting in missed opportunities to identify and modify coronary artery disease at younger ages and overstating the need to modify risk factors in “older” (>65 years) age. He cited studies demonstrating the early onset of atherosclerosis in Western societies. One study included 262 patients with heart transplants in which a monotonic progression of atheroma in the native coronary arteries related to patient age was found [Tuzcu EM et al. Circulation 2001]. The study indicated that by age 30 to 40 years, 50% to 60% of these individuals have atheroma. These data are supported by the Vietnam War study in which 45% of the 105 participants (mean age, 22 years) had arterial plaque [Joseph A et al. J Am Coll Cardiol 1993]. Finally, coronary artery calcium (CAC; a specific sign of atherosclerosis) has been found in 40% to 60% of individuals by age 45 years [Blaha MJ et al. Circ Cardiovasc Imaging 2014].
Michael J. Blaha, MD, MPH, Johns Hopkins University, Baltimore, Maryland, USA, sees clear forward steps in the prevention guidelines with the incorporation of race into the risk estimator, the inclusion of stroke into the composite outcome of the risk estimator, and the prioritization of statins. However, Dr. Blaha expressed concern about the validity of the risk estimator.
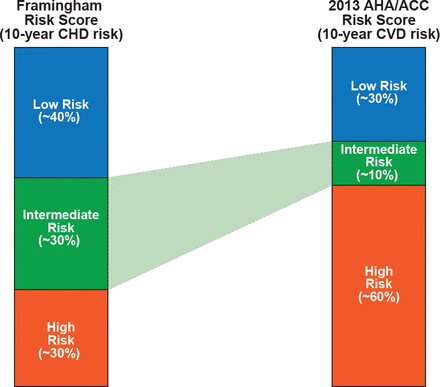
Dr. Blaha questioned whether the greater dependence on chronologic age in the new estimator provides any guidance with respect to prevention. The estimator calculates a >7.5% 10-year risk for patients with otherwise ideal parameters at approximately age 60 to 65 years in men and age 65 to 70 years in women. He also has concerns about the classification of patients with respect to risk, particularly those who might be at intermediate risk. Comparing Framingham Risk Score (FRS), to the new estimator would now classify significantly more patients previously considered “intermediate risk” as “high risk” (Figure 2). Dr. Blaha's concern with this shift is that it seems to imply more certainty with respect to classification, which in his opinion has not been validated. In addition, the risk estimator's dependence on age moves patients from intermediate to high risk based on age alone and more quickly (∼3 years vs ∼8 years) than the FRS, a well-validated risk estimator. Dr. Blaha's final concern with the new risk estimator was a concern for poor calibration, or overestimation of predicted risk.
Relative Allocation of High, Intermediate*, and Low Risk Patients: FRS Versus 2013 AHA/ACC Risk Estimator
Reproduced with permission from MJ Blaha, MD, MPH.
Harvey S. Hecht, MD, Mount Sinai Hospital, New York, New York, USA, discussed his concern that the 2013 guidelines have de-emphasized CAC in determination of who might be assigned statin therapy. Dr. Hecht believes that the evidence for the value of CAC in risk estimation is strong. He noted that numerous studies have demonstrated CAC to be an independent predictor of CV events. It holds a Class II (Level of Evidence A) recommendation in the 2010 ACCF/AHA guideline for assessment of CV risk in asymptomatic adults [Greenland P et al. Circulation 2010; J Am Coll Cardiol 2010] and the 2012 European guidelines on CV disease prevention in clinical practice [Perk I et al. Eur Heart J 2012]. In addition, multiple studies have shown a direct relationship between patients knowing their CAC scan results and improved adherence to therapy [Youssef G et al. Curr Cardiol Rep 2013; Orakzai RH et al. Am J Cardiol 2008; Taylor AJ et al. J Am Coll Cardiol 2008]. In one recent study, greater improvements in BP, LDL, waist size, weight, and FRS were found in patients with abnormal CAC scores, who were shown their results (Table 2) [Rozanski A et al. J Am Coll Cardiol 2011].
Effects of Coronary Artery Scanning on Outcomes in the EISNER Trial
Concerns that contributed to the authors lowering the recommendation for CAC were cost and increased potential radiation exposure. Dr. Hecht noted that the radiation level for CAC is now in the same range as mammography and decreasing. The cost has also dramatically decreased to about $100. While full consensus was not reached on the controversial topics discussed, the speakers appeared to agree that clinicians should not lose sight of the purpose of the guidelines, which is to guide decision-making, not mandate it.
The editors would like to thank the many members of the American College of Cardiology presenting faculty who generously gave their time to ensure the accuracy and quality of the articles in this publication.
Article Notes
-
↵* Intermediate risk for the ATP4 estimator assumes a score of 5 to 7.5. ACC=American College of Cardiology; AHA=American Heart Association; CHD=coronary heart disease; CVD=cardiovascular disease.
- © 2014 MD Conference Express®