Summary
Approximately 10% to 20% of patients treated with statins experience side effects, primarily musculoskeletal side effects, which diminish compliance or cause discontinuation of therapy [Zhang H et al. Ann Intern Med 2013; Mancini GB et al Can J Cardiol 2011]. Evolocumab, a fully human monoclonal antibody that binds proprotein convertase subtilisin/kexin type 9 (PCSK9), reduced levels of low-density lipoprotein cholesterol (LDL-C) to a greater extent than ezetimibe in hypercholesterolemic patients who could not tolerate effective doses of statins. This article presents the results from a double-blind multicenter Phase 3 Goal Achievement After Utilizing an Anti-PCSK9 Antibody in Statin-Intolerant Subjects 2 study [GAUSS-2; Stroes E et al. J Am Coll Cardiol 2014].
- Lipid Disorders
- Cardiology Clinical Trials
- Lipid Disorders
- Cardiology Clinical Trials
- Cardiology
Approximately 10% to 20% of patients treated with statins experience side effects, primarily musculoskeletal side effects, which diminish compliance or cause discontinuation of therapy [Zhang H et al. Ann Intern Med 2013; Mancini GB et al Can J Cardiol 2011]. Reduced adherence to, and discontinuation of, statins adversely affect survival in both the primary and secondary prevention settings [Chowdhury R et al. Eur Heart J 2013; Perreault S et al. Eur J Clin Pharmacol 2009; Rasmussen JN et al. JAMA 2007]. Further therapeutic efforts are therefore needed to lower low-density lipoprotein cholesterol (LDL-C) in this setting. Evolocumab, a fully human monoclonal antibody that binds proprotein convertase subtilisin/kexin type 9 (PCSK9), reduced levels of low-density lipoprotein cholesterol (LDL-C) to a greater extent than ezetimibe in hypercholesterolemic patients who could not tolerate effective doses of statins.
Erik Stroes, MD, Academic Medical Center, Amsterdam, The Netherlands, presented the results from a double-blind multicenter Phase 3 Goal Achievement After Utilizing an Anti-PCSK9 Antibody in Statin-Intolerant Subjects 2 study [GAUSS-2; Stroes E et al. J Am Coll Cardiol 2014] in which 307 patients with hypercholesterolemia who were statin intolerant were randomized on a 2:2:1:1 basis to evolocumab, 140 mg Q2W or 420 mg QM plus daily oral placebo, or subcutaneous placebo (Q2W or QM) plus 100 mg/day of oral ezetimibe. The study was designed to build on the Phase 2 experience with evolocumab, which demonstrated potent LDL-C lowering in hypercholesterolemic patients intolerant to at least one statin [Sullivan D et al. JAMA 2012].
Participants qualified for the study if they were unable to tolerate effective doses of ≥2 statins because of myalgia, myopathy, myositis, or rhabdomyolysis that resolved with statin discontinuation [Stroes E et al. J Am Coll Cardiol 2014]. Their mean LDL-C at baseline was ∼195 mg/dL. The coprimary endpoints were the mean percent change from baseline in LDL-C at Week 12 and the mean at Weeks 10 and 12.
Mean age of patients ranged from 60 to 63 years in the four treatment groups. More than 90% were white and the distribution between males and females was fairly equal. About 60% of patients qualified as high risk under the National Cholesterol Education Program risk category system. An additional 15% were classified as moderate risk. More than half of the patients were intolerant to at least three statins. Seventy eight percent to 88% had myalgia as their worst muscle-related side effect to statins.
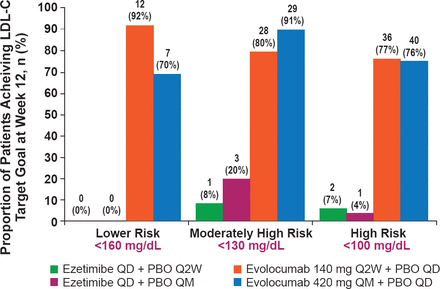
Compared with ezetimibe, patients randomized to evolocumab Q2W had a 37% reduction in LDL-C at a mean of 10 and 12 weeks, and a 38% reduction at 12 weeks. Patients randomized to monthly evolocumab had a 39% reduction in LDL-C at a mean of 10 and 12 weeks and a 38% reduction at 12 weeks as compared with ezetimibe (p<0.001 for all comparisons). Compared with baseline, the mean reductions in LDL-C at 12 weeks were 56% with Q2W evolocumab and 53% with monthly dosing. Of evolocumab-treated patients at high risk, >75% achieved LDL-C <100 mg/dL compared with <10% of ezetimibe-treated patients (Figure 1).
LDL-C Goal Achievement at Week 12
LDL-C=low-density lipoprotein cholesterol.
Reproduced from Stroes E et al. Anti-PCSK9 Antibody Effectively Lowers Cholesterol in Patients with Statin Intolerance: The GAUSS-2 Randomized, Placebo-controlled Phase 3 Clinical Trial of Evolocumab. J Am Coll Cardiol 2014 doi: 10.1016/j.jacc.2014.03.019. With permission from Elsevier.
Both dosing frequencies of evolocumab also significantly reduced levels of apolipoprotein B and lipoprotein (a) and increased levels of high-density lipoprotein cholesterol (HDL-C) and apolipoprotein A-I.
The rate of adverse events was generally balanced across treatment groups. The most common adverse events (>5% in evolocumab combined group) were headache (8% with evolocumab vs 9% with ezetimibe), myalgia (8% vs 18%), pain in extremity (7% vs 1%), and muscle spasms (6% vs 4%).
Dr. Stroes noted that the robust LDL-C lowering and good tolerability suggests that evolocumab is a promising therapy for high-risk hypercholesterolemic patients.
- © 2014 MD Conference Express®