Summary
The monoclonal antibody evolocumab (previously AMG 145), which inhibits proprotein convertase subtilisin/kexin type 9 (PCSK9), dramatically lowered levels of low-density lipoprotein cholesterol (LDL-C) in Phase 2 clinical trials when administered alone or in combination with a statin, including among patients intolerant of statin therapy, those with familial hypercholesterolemia, and in those treated in a 52-week study [Koren MJ et al. Circulation 2013; Giugliano RP et al. Lancet 2012; Koren MJ et al. Lancet 2012; Raal F et al. Circulation 2012; Sullivan D et al. JAMA 2012]. This article discusses the Phase 3 LDL-C Assessment With PCSK9 Monoclonal Antibody Inhibition Combined With Statin Therapy-2 study [LAPLACE-2; NCT01763866].
- Cardiology Clinical Trials
- Lipid Disorders
- Cardiology Clinical Trials
- Lipid Disorders
- Cardiology
A fully human monoclonal antibody that inhibits proprotein convertase subtilisin/kexin type 9 (PCSK9), when added to background statin therapy, reduces levels of low-density lipoprotein cholesterol (LDL-C) in patients with hypercholesterolemia and mixed dyslipidemia.
The monoclonal antibody evolocumab (previously AMG 145), which inhibits PCSK9, dramatically lowered levels of LDL-C in Phase 2 clinical trials when administered alone or in combination with a statin, including among patients intolerant of statin therapy, those with familial hypercholesterolemia, and in those treated in a 52-week study [Koren MJ et al. Circulation 2013; Giugliano RP et al. Lancet 2012; Koren MJ et al. Lancet 2012; Raal F et al. Circulation 2012; Sullivan D et al. JAMA 2012]. These observations led to the randomized, multicenter, placebo-controlled, double-blind Phase 3 LDL-C Assessment With PCSK9 Monoclonal Antibody Inhibition Combined With Statin Therapy-2 study [LAPLACE-2; NCT01763866], the results of which were presented by Jennifer G. Robinson, MD, MPH, University of Iowa, Iowa City, Iowa, USA.
The efficacy and safety of evolocumab were assessed in 1896 patients with primary hypercholesterolemia and mixed dyslipidemia (LDL-C ≥80 mg/dL) who were also taking a high- or moderate-intensity statin. Patients were eligible for the study if they had a central laboratory fasting LDL-C at screening of ≥150 mg/dL (4.0 mmol/L; no statin at screening), ≥100 mg/dL (2.6 mmol/L; nonintensive statin at screening), or ≥80 mg/dL (2.1 mmol/L; intensive statin at screening). The primary objective of this study was to evaluate the efficacy (vs placebo) of 12 weeks of subcutaneous (SC) evolocumab administered every 2 weeks or every month when used in combination with a daily statin with or without ezetimibe on percent change from baseline in LDL-C.
Patients were initially randomized to high (atorvastatin 80 mg or rosuvastatin 40 mg) or moderate (atorvastatin 10 mg, rosuvastatin 5 mg, or simvastatin 40 mg) intensity statin therapy. Following a 4-week stabilization period, patients randomized to atorvastatin 10 or 80 mg were then randomized to 1 of 6 treatment groups: SC evolocumab 140 mg Q2W and oral placebo QD; SC evolocumab (420 mg) QM and oral placebo QD; SC placebo Q2W and oral placebo QD; SC placebo QM and oral placebo QD; SC placebo Q2W and ezetimibe 10 mg QD; or SC placebo QM and ezetimibe 10 mg QD. Patients randomized to rosuvastatin or simvastatin were then randomized to 1 of 4 treatment groups: evolocumab Q2W, evolocumab QM, SC placebo Q2W, or SC placebo QM.
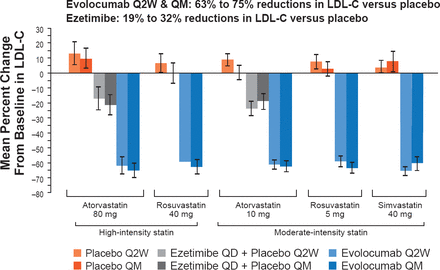
Overall, 1896 patients were randomized, mean patient age was 60 years, ∼20% had coronary artery disease, ∼10% had peripheral arterial disease or cerebrovascular disease, and ∼16% had type 2 diabetes. Their mean baseline LDL-C was ∼110 mg/dL (2.85 mmol/L). When combined with either a high- or moderate-intensity statin, evolocumab-treated groups showed highly significant reductions in LDL-C versus placebo of 63% to 75% (Figure 1). Compared with placebo, ezetimibe when combined with atorvastatin reduced levels of LDL-C by 19% to 32%. An LDL-C level <70 mg/dL was achieved by 86% to 94% of evolocumab recipients on a moderate-intensity statin and 93% to 95% on a high-intensity statins.
LDL-C Response at Mean of Weeks 10 and 12
LDL-C=low-density lipoprotein cholesterol.
Reproduced with permission from JG Robinson, MD, MPH.
Adding evolocumab to moderate-intensity statin regimens reduced LDL-C levels to a mean of 38 to 45 mg/dL (0.98 to 1.16 mmol/L), and to 35 to 38 mg/dL (0.09 to 0.98 mmol/L) with high-intensity statin regimens.
Compared with placebo, evolocumab also significantly reduced levels of non-high-density lipoprotein cholesterol by 58% to 65%, apolipoprotein B by 51% to 59%, and lipoprotein (a) by 21% to 36%.
There were no notable differences in safety and tolerability in evolocumab-, placebo-, and ezetimibe-treated patients.
- © 2014 MD Conference Express®