Summary
Although natalizumab has high efficacy in patients with multiple sclerosis (MS), a risk of developing progressive multifocal leukoencephalopathy (PML) has been identified in patients exposed to it. PML is a concerning adverse event of natalizumab, as PML can cause substantial morbidity or mortality in MS patients. This article discusses the management of PML in patients with MS, the prevalence of John Cunningham virus reactivation during treatment with natalizumab, as well as the use of T2*-weighted high-resolution MRI to detect PML
- Demyelinating Diseases
- Neurology Genomics
- Neuroimaging
- Neurology
- Demyelinating Diseases
- Neurology Genomics
- Neuroimaging
Although natalizumab has high efficacy in patients with multiple sclerosis (MS), a risk of developing progressive multifocal leukoencephalopathy (PML) has been identified in patients exposed to it. PML is a concerning adverse event of natalizumab, as PML can cause substantial morbidity or mortality in MS patients. Ralf Gold, MD, Ruhr-University Bochum, Bochum, Germany, discussed recent updates in the management of PML in patients with MS.
Although natalizumab has high clinical and magnetic resonance imaging (MRI) efficacy for > 2 years [Havrdova E et al. Lancet Neurol. 2009], exposure to it results in a risk of developing PML at a rate of about 80 patients per year. Almost 50% of patients who develop PML will present with cognitive impairment, whereas 30% to 40% will present with motor weakness and speech deficits and about 25% present with visual deficits [Berger JR. Cleve Clin J Med. 2011].
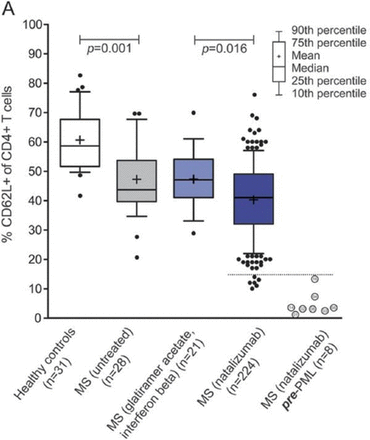
A recent study suggests that L-selectin may serve as a biomarker indicating that a patient with MS may be at a greater risk of developing PML if treated with natalizumab [Schwab N et al. Neurology. 2013]. In this international study, patients treated with long-term natalizumab demonstrated significantly lower levels of L-selectin-expressing CD4+ T cells as compared with patients who were not treated with it (40.2% vs 47.2%; P = .016) and healthy subjects (61%; P < .0001). In addition, the lowest percentage of L-selectin-expressing CD4+ T cells was associated with a 9-fold greater risk of developing PML (Figure 1).
L-selectin as a Potential Biomarker for PML Risk
MS, multiple sclerosis; PML, progressive multifocal leukoencephalopathy.
Schwab N et al. Selectin is a possible biomarker for individual PML risk in natalizumab-treated MS patients. Neurology. 2013;81:865–871.
In a study that evaluated the value of corticosteroid treatment of PML, corticosteroids administered to patients with relapsing MS had a profound impact on the virus-specific T-cell response, especially on John Cunningham virus (JCV), resulting in a decrease of T-cell proliferation induced by Epstein-Barr virus, influenza, and JCV, as well as interferon γ and tumor necrosis factor a-producing JCV-specific CD8+ T cells. These results suggested that corticosteroids should not be given before the onset of clinical or radiologic signs of PML-immune reconstitution inflammatory syndrome and thus should be used to treat but not to prevent it [Antoniol C et al. Neurology. 2012]. Another study found that 53% of patients with natalizumab-associated PML developed seizures, primarily as status epilepticus, with seizure onset occurring on average about 61 days after onset of natalizumab-associated PML. Seizures were associated with immune reconstitution inflammatory syndrome in a number of patients [Hoepner R et al. Ther Adv Neurol Disord. 2014]. Prof Gold noted that the algorithm at his institution is to administer preventive antiepileptic therapy. In some cases, “spillover” occurs in which patients who are treated with a new therapy (eg, dimethyl fuma-rate) but who had previously received natalizumab develop PML.
The functional status of patients who survive PML is most commonly that of moderate impairment, in which they are unable to work but are still able to care for themselves [Vermersch P et al. Neurology. 2011]. In this study, the survival rate was about 71%; in a study of patients at Bochum Hospital, the survival rate was 96%.
Spyridon Chalkias, MD, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA, discussed the prevalence of JCV reactivation during treatment with natalizumab. The purpose of his study was to determine the prevalence of JCV reactivation (including the effect of duration of natalizumab treatment), early JCV reactivation events, and the specific immune response [Chalkias S et al. Ann Neurol. 2014]. Samples of blood, cerebrospinal fluid (CSF), and urine were collected from patients with MS who were JCV seropositive [Chalkias S et al. Ann Neurol. 2014]. Although JCV DNA was detected in the CSF of several patients after 18 months of natalizumab therapy, they did not have clinical or MRI symptoms of PML, suggesting that JCV DNA found in the CFS does not necessarily mean that the patient has PML. In addition, JCV is associated with peripheral blood mononuclear cells (PBMCs), with a particularly high viral load in CD34+ cells and monocytes. A JCV-specific CD4+ T-cell response is associated with JCV in CD34+ cells and B cells. Therefore, the results of this study indicate that JCV that is present within PBMCs may serve as a surrogate marker of JCV reactivation.
Varun Sethi, MD, National Institute of Neurological Disorders and Stroke, Bethesda, Maryland, USA, discussed the use of T2*-weighted high-resolution MRI to detect PML. PML lesions can be detected in conventional MRI; in T2-weighted images, PML lesions have hyperintense signal, whereas in T1-weighted images, the signal is hypointense [Khoury MN et al. Ann Neurol. 2014].
In a cohort of 12 patients with PML, 5 of whom had MS (following treatment with natalizumab for a mean duration of 5 years), PML lesions appeared on T2*-weighted images as asymmetric white matter hyperintensities with an adjacent hypointense band at the cortical border. Preliminary studies based on MRI-based quantitative susceptibility mapping suggest that the band is caused by a paramagnetic substance; histopathologic correlation according to diaminobenzidine-enhanced Perl's stain demonstrated the presence of iron. Interestingly, iron is required for myelination by oligodendrocytes, the same cells that are infected by JCV [Todorich B et al. Glia. 2009]. However, the mechanism by which iron accumulates at this location in the PML lesion is not yet known. Dr Sethi further indicated that the results of this study suggest that the presence of a hypointense band in T2*-weighted images may aid the diagnosis of a PML-like lesion that involves the cortex.
In conclusion, early identification of PML is important as early treatment may improve outcomes. Potential biomarkers have been identified in PBMCs and T2-weighted MRI that may aid clinicians in the diagnosis of this potentially devastating complication of natalizumab therapy.
- © 2014 MD Conference Express®