Summary
Substrate-based ablation of ventricular tachycardia (VT) in patients with ischemic cardiomyopathy resulted in fewer VT recurrences and less rehospitalization compared with the conventional clinical VT ablation. This article present data from the Ablation of Clinical Ventricular Tachycardia Versus Addition of Substrate Ablation on the Long-term Success Rate of VT Ablation trial [VISTA; NCT01045668].
- Cardiology Clinical Trials
- Interventional Techniques & Devices
- Inflammatory Disease
- Cardiology Clinical Trials
- Cardiology & Cardiovascular Medicine
- Interventional Techniques & Devices
- Inflammatory Disease
Substrate-based ablation of ventricular tachycardia (VT) in patients with ischemic cardiomyopathy resulted in fewer VT recurrences and less rehospitalization compared with the conventional clinical VT ablation. Luigi Di Biase, MD, PhD, Texas Cardiac Arrhythmia Institute, Austin, Texas, USA, and Albert Einstein College of Medicine, Bronx, New York, USA, presented data from the Ablation of Clinical Ventricular Tachycardia Versus Addition of Substrate Ablation on the Long-term Success Rate of VT Ablation trial [VISTA; NCT01045668].
Patients with ischemic cardiomyopathy and stable monomorphic VT may undergo catheter ablation as an option to reduce implantable cardiac defibrillator shocks. However, it is unclear if ablation of the clinical stable VT or more extensive substrate-based ablation is more beneficial. The purpose of the VISTA trial was to determine whether substrate-based ablation improved outcomes compared with the conventional ablation of the stable clinical VTs.
In the open-label, randomized, parallel-group multicenter VISTA trial, 128 patients with symptomatic, drug-refractory, hemodynamically stable VTs after coronary artery disease were randomly assigned to undergo clinical stable VT ablation or substrate ablation. Every 3 months, patients were assessed by implantable device interrogations and examination during office visits. Baseline characteristics were similar between both study arms, with the mean age ranging from 65 to 67 years and with most patients being men (93%) with hypertension (72% to 76%) or diabetes (32% to 42%). In addition, the mean left ventricular ejection fraction (LVEF) was 32% to 33%, and 33% to 35% had previously undergone coronary artery bypass graft surgery.
The primary endpoint of the VISTA trial was recurrence of any VTs over the 12-month period following ablation. Recurrence was defined as any arrhythmia that required device-based treatment or any VT event that occurred during clinical evaluation. The secondary end points included periprocedural complications and postprocedural mortality and rehospitalization at 12 months.
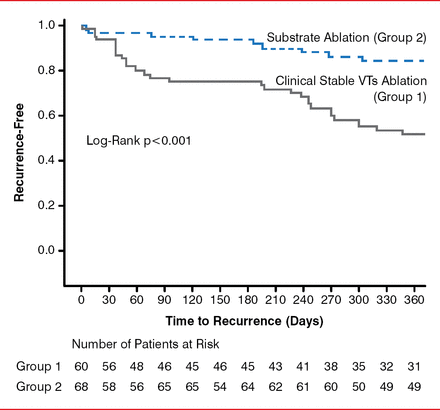
At 12 months, 51.7% of patients who underwent clinical VT ablation achieved freedom from any recurrent VTs, compared with 84.5% of patients who underwent substrate ablation (log-rank p<0.001; Figure 1). In addition, significantly more patients who underwent clinical VT ablation required rehospitalization (32%) than those patients who underwent substrate ablation (12%; p=0.014). Overall mortality in the VISTA trial was 11.9%, with mortality occurring in 15% of patients who underwent clinical VT ablation and 8% who underwent substrate ablation (p=0.21).
VT Recurrence-Free Survival After Ablation
VT=ventricular tachycardia.
Reproduced with permission from L Di Biase, MD, PhD.
In addition, use of the clinical VT ablation method was associated with a greater rate of VT recurrence, with a hazard ratio of 3.84 (p=0.001). Interestingly, other risk factors that were associated with VT recurrence were diabetes (HR, 3.11; p=0.02), LVEF (HR, 0.77; p=0.035), electrical storm (HR, 1.86; p=0.043), male sex (HR, 3.23; p=0.029), and age per 5-year increase (HR, 1.11; p=0.016). Clinical VT ablation (HR, 3.1; p=0.01) and diabetes (HR, 2.75; p=0.042) remained independent predictors of VT recurrence after adjustment for covariates based on a Cox multivariate model.
Complications of ablation included 1 atrial valve fistula in the clinical VT ablation group and 5 pericardial effusions (2 in the clinical VT ablation group and 3 in the substrate ablation group).
Dr. Di Biase stated that, in his opinion, the data from the randomized VISTA trial indicate that substrate-based ablation may be superior to clinical VT ablation in patients with ischemic cardiomyopathy with stable VT. However, he noted that additional studies are needed to confirm the results of the VISTA trial.
- © 2014 MD Conference Express®