Summary
Atrial fibrillation (AF) is associated with a 4- to 5-fold increased risk of stroke, a doubling of the risk for dementia, a tripling of the risk for heart failure (HF), and a 40% to 90% increased risk of overall mortality [Benjamin EJ et al. Circulation 2009]. By 2050, it is estimated that the number of persons with AF in the United States will exceed 10 million [Miyasaka Y et al. Circulation 2006]. This article discusses the relationship between the left atrial appendage (LAA) and AF.
- Arrhythmias
- Cerebrovascular Disease
- Interventional Techniques & Devices
- Cardiology & Cardiovascular Medicine
Atrial fibrillation (AF) is associated with a 4- to 5-fold increased risk of stroke, a doubling of the risk for dementia, a tripling of the risk for heart failure (HF), and a 40% to 90% increased risk of overall mortality [Benjamin EJ et al. Circulation 2009]. By 2050, it is estimated that the number of persons with AF in the United States will exceed 10 million [Miyasaka Y et al. Circulation 2006]. During a special session, speakers discussed the relationship between the left atrial appendage (LAA) and AF.
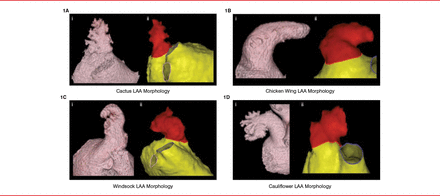
The LAA is the site of 91% of AF-related left-atrial thrombi; thus, understanding its role is essential to understanding stroke risk. Luigi Di Biase, MD, PhD, St David's Medical Center, Austin, Texas, USA, discussed a study of LAA morphology in patients with a history of stroke or transient ischemic attack (TIA) to determine whether there was a correlation with stroke risk [Di Biase L et al. J Am Coll Cardiol 2012]. The study included 932 patients with drug refractory AF who were scheduled for ablation. All patients underwent computed tomography and magnetic resonance imaging to categorize the LAA by shape (Table 1; Figure 1).
Description of LAA Morphologic Classifications
CT and MRI Scans of Various LAA Morphologies
CT=computed tomography; LAA=left atrial appendage; MRI=magnetic resonance imaging.
Reproduced from Di Biase L et al. Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from a multicenter study. J Am Coll Cardiol 2012;60:531–538. With permission from Elsevier.
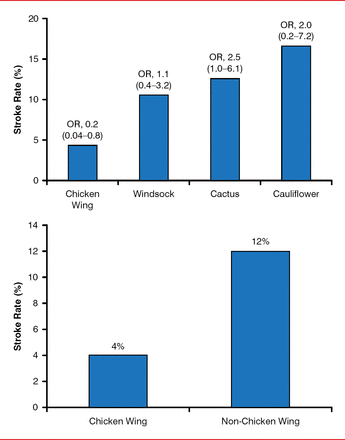
After controlling for CHADS2 score, sex, and AF types, non-chicken wing morphology was an independent predictor of stroke (OR, 2.95; 95% CI, 1.75–4.99; p=0.041; Figure 2), possibly due to the extent of trabeculation.
Prior Stroke or TIA Event Rate by Morphology
TIA=transient ischemic attack.
Reproduced from Di Biase L et al. Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from a multicenter study. J Am Coll Cardiol 2012;60:531–538. With permission from Elsevier.
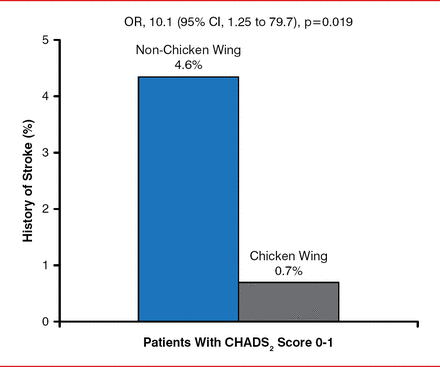
LAA morphology was found to be particularly important among patients with a CHADS2 score of 0 or 1. For those patients, having non-chicken wing morphology increased the risk of prior stroke or TIA more than 6-fold versus chicken wing (Figure 3). These results could affect anticoagulation and occlusion device management of patients with AF, especially those with a low to intermediate stroke risk.
Impact of Non-Chicken Wing Mortality in Patients With CHADS2 Score 0 or 1
Reproduced from Di Biase L et al. Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from a multicenter study. J Am Coll Cardiol 2012;60:531–538. With permission from Elsevier.
Several case reports have recently traced the arrhythmogenic origins of atrial tachycardia to the LAA [Yang Q et al. Europace 2012; Benussi S et al. Circulation 2011], which is also the origin of a significant proportion of localized reentrant atrial tachycardia [Hocini M et al. Heart Rhythm 2011; Di Biase L et al. Circulation 2010]. An important source of the arrhythmogenic properties of the LAA is its anatomy, which can consist of extensive trabeculation that influences wave propagation and favors the formation of a conduction block or slow conduction and the initiation of reentry [Cabrera JA et al. Eur Heart J 2008].
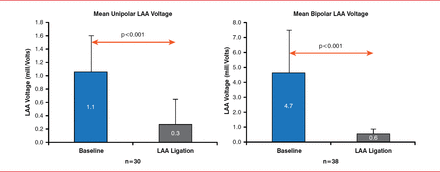
Dhanunjaya Lakkireddy, MD, University of Kansas Hospital, KU Cardiovascular Research Institute, Kansas City, Kansas, USA, discussed the role of LAA exclusion as an adjunct strategy for AF ablation. Establishing normal sinus rhythm in patients with AF can reduce their stroke rate to that of patients without AF regardless of CHADS2 score [Bunch TJ et al. Heart Rhythm 2013]; however, the 5-year success rate of catheter ablation among patients with long-standing persistent AF is poor (ie, ∼20% after one procedure and 45% after multiple procedures) [Tilz RR et al. J Am Coll Cardiol 2012]. Adjunctive treatment to exclude the LAA can improve outcomes. Electrical isolation of the LAA is associated with a reduction in the overall burden of AF at 3 months [Afzal R et al. In press] and improvements in short- and long-term outcomes of AF therapies [Di Biase L et al. Circulation 2010]. LAA exclusion with either a clip [Starck CT et al. Interact Cardio Vasc Thorac Surg 2012] or the LARIAT device [Han FT et al. Heart Rhythm 2014] results in ischemic necrosis of the LAA and an acute reduction in LAA voltage (Figure 4).
Effects of LAA Ligation on LAA Electrical Activity
LAA=left atrial appendage.
Reproduced from Han FT et al. The effects of LAA ligation on LAA electrical activity. Heart Rhythm 2014;11:864–870. With permission from Elsevier.
Dr. Lakkireddy presented data from patients who were enrolled in the Left Atrial Appendage Ligation and Ablation for Persistent Atrial Fibrillation Registry [LAALA-AF; Lakkireddy N et al. J Am Coll Cardiol 2014 (abstr 1182–111)]. Patients had persistent AF and underwent a LARIAT procedure, followed by AF ablation. Dr. Lakkireddy compared them with a control population matched for age, sex, and AF type that was undergoing only ablation. After the LARIAT procedure, the LAA was completely eliminated. In addition, the LA volume was considerably lower, leaving less tissue available for the propagation and perpetuation of AF. Compared with patients receiving ablation only, patients undergoing LARIAT plus ablation had less recurrence of AF for 1 year after their first ablation and were less likely to require repeat ablation (Table 2).
Outcomes in the LAALA-AF Registry, n (%)
LAA exclusion also affects the neuroendocrine function. In the Impact of Left Atrial Appendage Ligation on Systemic Homeostasis study [LAA Homeostasis], N-terminal proatrial natriuretic peptide and brain natriuretic peptide levels dropped acutely after exclusion of the LAA but returned to preprocedure levels after ∼24 hours and remained there at ∼3 months. Adrenaline, noradrenaline, aldosterone, and renin levels also dropped after the procedure but remained lower at 3 months. LAA electrical exclusion benefits patients with atrial arrhythmia by eliminating the triggers and reentry, reducing arterial volume and substrate, and altering the renin-angiotensin-aldosterone system.
In the United States, there are no devices for use in patients with AF currently approved for LAA closure to prevent stroke; however, several devices are approved in Europe. Several other devices are about to begin trials in the United States. Vivek Y. Reddy, MD, Mount Sinai Hospital, New York, New York, USA, discussed some of these devices, with an emphasis on the Watchman device. Most data for the Watchman device comes from the Watchman LAA System for Embolic Protection in Patients With Atrial Fibrillation study [PROTECT-AF; Reddy V et al. Circulation 2013], the Evaluation of the Watchman LAA Closure Device in Patients With Atrial Fibrillation Versus Long-Term Warfarin Therapy study [PREVAIL; NCT01182441], and the Continued Access Protocol Registry [CAP; Reddy VY et al. Circulation 2011].
After 4 years of follow-up in the PROTECT-AF trial, the Watchman device was superior to warfarin on the primary efficacy end point of stroke, embolization, or death (rate ratio [RR], 0.60; 95% CI, 0.41–1.05; p=0.960 for superiority). Subjects receiving the Watchman device had 32% fewer strokes, driven largely by a significant reduction in hemorrhagic stroke (RR, 0.15; 95% CI, 0.03–0.49; p=0.999 for superiority). Compared with warfarin, there were 60% fewer cardiovascular deaths in the group treated with the Watchman device and a 34% reduction in all-cause mortality (p=0.0379; Table 3) [Reddy V et al. HRS 2013 (abstr LB01–03)].
PROTECT-AF: Primary Efficacy End Point
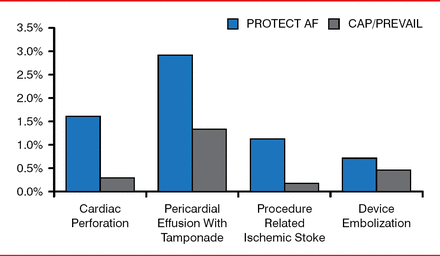
The overall rate of safety-related events was not significantly different in the 2 groups. Although there were early procedural-related complications in the Watchman group, the safety profile improved in successive studies (Figure 5).
Safety: PROTECT-AF Versus CAP/PREVAIL
Note: Overembolization rate across studies is 0.5%.
Reproduced with permission from VY Reddy, MD.
The ASA Plavix Registry [ASAP; Reddy V et al. J Am Coll Cardiol 2013] was designed to assess whether LAA closure is safe without an initial warfarin transition, and it included 150 patients (mean CHADS2 score, 2.8) with a strong contraindication to warfarin who received only aspirin and clopidogrel. Subjects were followed for 14.4 months, at which point the observed stroke rate was 1.7%, compared with an expected rate of 7.3% based on CHADS2 score.
Most data for the other LAA closure devices come from nonrandomized short-term studies published only in abstract form; however, the results are promising. These include the Amplatzer Cardiac Plug [Walsh K et al. Euro-PCR 2012 (abstr); Park JW et al. J Invasive Cardiol 2009] and the Wavecrest Device [Reddy V et al. HRS 2014 (abstr)]. All the devices have some rate of leak. The clinical significance of this is currently unknown, although, at least for the Watchman device, it does not seem to be associated with adverse outcomes [Viles-Gonzales J, Reddy V. J Am Coll Cardiol 2012]. The new devices are promising, but additional prospective trials are needed, and their use in the setting of the novel oral anticoagulants has not yet been determined.
Samuel J. Asirvatham, MD, Mayo Clinic, Rochester, Minnesota, USA, provided guidance on understanding and managing complications related to LAA exclusion. One source of complications in LAA exclusion procedures is the anterior location of the LAA. Epicardial access is complicated by the fact that the LAA is hidden in the right anterior oblique view and that, in the left anterior oblique view, it is a very superior structure that lies alongside the pulmonary artery.
Potential external complications include the position of the left anterior descending artery, which is more important for an epicardial approach, and the location of the circumflex, which may be a limiting factor for any procedure that requires placement of a device at the ostium. The phrenic nerve is also close to the LAA and must be considered with performing combined procedures.
There is no standard approach if a thrombus is recognized during the procedure. Dr. Asirvatham recommends placing filters in both the carotids, then (depending on its size) using the retrieval device or suction to remove the thrombus. To reduce the possibility of thrombus development, he suggests using heparin before the transseptal puncture. Air is also a potential issue, and any large-sheath exchanges should be done underwater or with continuous flush running. The first indication of a pericardial effusion with an appendage procedure will be around the appendage. Dr. Asirvatham recommends positioning an ultrasound probe in that area.
The incidence of device embolization, a particularly feared complication, can be reduced with adequate hydration, proper placement (avoid tilting), and adequate compression.
- © 2014 MD Conference Express®