Summary
The assessment of disease activity and burden has evolved from one based on clinical activity to one based on radiologic criteria (eg, number of new/enlarging T2 lesions, number of gadolinium-enhancing [Gd+] lesions, and brain atrophy/black holes). Treatment has moved from slowing disease progression to treatments (eg, natalizumab, fingolimod, interferon beta-1b [IFN-β-1b]) that improve and sustain function.
- Demyelinating Diseases
- Neurology
- Demyelinating Diseases
The assessment of disease activity and burden has evolved from one based on clinical activity to one based on radiologic criteria (eg, number of new/enlarging T2 lesions, number of gadolinium-enhancing [Gd+] lesions, and brain atrophy/black holes). Treatment has moved from slowing disease progression to treatments (eg, natalizumab, fingolimod, interferon beta-1b [IFN-β-1b]) that improve and sustain function. Hans-Peter Hartung, MD, Heinrich-Heine-University, Düsseldorf, Germany, believes that multiple sclerosis (MS) treatment needs to eventually evolve to the point where not only function is improved but disease damage is also repaired.
FREEDOM FROM DISEASE ACTIVITY
An important goal of new treatments is freedom from disease activity, defined as being free of relapses and disease progression and the absence of Gd+ or T2 lesions [Hartung HP, Aktas O. Lancet Neurol. 2011]. An example of such a treatment is natalizumab, an adhesion-molecule inhibitor that decreases the risk of sustained progression of disability and the rate of clinical relapse in patients with relapsing-remitting MS (RRMS) [Polman CH et al. N Engl J Med. 2006]. In this study on natalizumab for the treatment of MS, patients who received the drug were significantly less likely to relapse at 1 and 2 years (P < .001). Post hoc analyses of data from the AFFIRM study [Havrdova E et al. Lancet Neurol. 2009] showed that it was possible to significantly (P < .0001) reduce clinical disease activity in patients taking natalizumab, suggesting that disease remission is an obtainable goal. These findings have been repeated with fingolimod, alemtuzumab, and other modern agents in previously treated and treatment-naïve patients with RRMS.
Although these new immunomodulatory treatments have transformed the management of patients with RRMS, there has been no consistent benefit for progressive MS. To address this challenge, clinical trials are being designed to offer reliable measurement of clinical progression, take into account the natural history of progressive MS, and provide a clear role for imaging. Studies need to measure relapse rates, Expanded Disability Status Scale (EDSS) scores, T2 and Gd+ lesions, and brain atrophy—all of which correlate with disease progression.
According to Prof Hartung, an MS treatment that results in no annual brain volume loss, no sustained disability progression, no relapses, and no magnetic resonance imaging (MRI) activity is the goal. Possible future strategies for treating progressive MS include neuroprotection and remyelination.
TREATMENT REGIMENS FOR PATIENTS WITH ONGOING MS DISEASE ACTIVITY
Predictors for identifying patients in various stages of MS disease activity are needed to optimize treatment regimens. Xavier Montalban, MD, Hospital Universitari Vall d'Hebron, Barcelona, Spain, spoke about how early disease activity indicators might be used. The Rio scoring system, used to assess treatment failure, uses relapse (≥ 1) in first 12 months, increases in EDSS score, and T2 or Gd+ MRI-detected lesions (≥ 3 active) to measure disease activity [Río J et al. Mult Scler. 2009]. The risk of MS activity after 3 years significantly increases with 2 positive variables (OR, 5.9; 95% CI, 2.5 to 15.6; P < .0001) and 3 positive variables (OR, 13.2; 95% CI, 2.9 to 1 2 5 .7; P = .0003) but not 1 positive variable (OR, 1.4; 95% CI, 0.7 to 2.6; P = .30).
MS activity during the first year of treatment with IFN-β-1b predicts poor outcomes in the long term (out to about 16 years) [Dobson R et al. Neurology. 2014]. Early MRI activity (increased number of new hyperintense T2 lesions and the presence of Gd+ lesions) appears to predict both future poor outcomes and progression defined by the EDSS score in patients with RRMS despite treatment with IFN-β-1b [Bermel RA et al. Ann Neurol. 2013]. Accuracy improves significantly when looking for Gd+ lesions (P < .001) rather than just new T2 or enlarging lesions (P = .080).
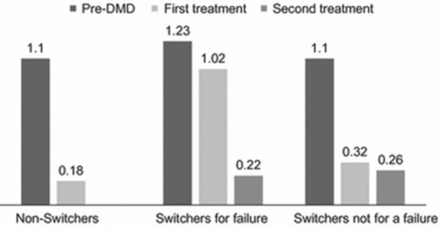
A considerable proportion of treated patients will develop a suboptimal response with first-line disease-modifying drugs (DMDs) and switch to other treatment regimens [Río J et al. Eur J Neurol. 2012]. Patients who switch to another DMD because of failed or suboptimal response with their first therapy may experience better outcomes with a second-line DMD. This is also true for patients who switch for reasons not based on failure (Figure 1).
Multiple Sclerosis Patients Who Switch DMDs Can Reduce Clinical Activity
DMD, disease-modifying drug.
Rio J et al. Change in the clinical activity of multiple sclerosis after treatment switch for suboptimal response. Eur J Neurol. 2012;19:899–904.
In one study, escalation to natalizumab seemed more effective than switching between IFN-β-1b or glatiramer acetate (GA) after 24 months [Prosperini L et al. Mult Scler. 2012]. In patients switching from IFN-β-1b to fingolimod or GA, fingolimod resulted in significantly fewer relapses when compared with GA (P = .0013) [Bergvall N et al. PLoS One. 2014]. Fingolimod reduced the rate of relapse with sustained benefits > 4.5 years.
According to Prof Montalban, other reasons why MS patients switch treatments include side effects, convenience, and cost. The decision to switch should be a shared decision with the patient's experiences, values, and preferences combined with the clinician's treatment options and knowledge of the drug's known potential benefits, harms, and outcomes.
COPOUSEP: FINAL EFFICACY AND SAFETY RESULTS
E. Le Page, MD, CHU Pontchaillou, Rennes, France, reported that oral dosing of methylprednisolone (MP) is noninferior to intravenous (IV) dosing for treatment of relapsing MS. Oral dosing may offer more patient comfort, simplified organization, and economic benefits.
COPOUSEP [NCT00984984] was a noninferior double-blind randomized trial conducted in 13 French centers. The objective was to evaluate oral versus IV MP on MS relapse. The primary end point was the number of patients who improved at day 28. Secondary outcomes were to compare the following: recovery at 3, 8, and 180 days; time to total recovery; and number of new recurrences up to 6 months after inclusion. An increase of ≥ 1 point on one or several Kurtzke functional systems defined a relapse. In addition, the most affected functional system had to achieve a score ≥ 2 points except for the “sensory or sensitive” function, which had to attain a score ≥ 3. Patients aged 18 to 55 years with RRMS (defined according to MacDonald criteria) and an EDSS of 0 to 5 before relapse were included in this study.
Patients (n = 200) were randomized to oral or IV MP (1 g/d). There were no significant group differences in baseline characteristics or MS activity. Most patients were women; the mean age was 35 years; the median MS duration was 5.9 years; and the mean EDSS was 3.4.
In the intention-to-treat (ITT) analysis at day 28, the proportion of improved patients was 77.7% in the MP IV group and 77.9% in the MP oral group (difference, +0.002; 95% CI, −0.095 to 0.10), validating the noninferiority hypothesis. The ITT clinical outcomes at day 28 (retreatment, total recovery, improved EDSS) and at 6 months (total recovery, median time to total recovery, relapse, starting or changing DMD) were similar in each group (P = NS). Two adverse events were more frequent in the oral MP-treated patients: insomnia (P < .02) and agitation (P < .04). Tolerance, clinical parameters, and effectiveness were similar between oral and IV MP.
FACTORS THAT DETERMINE DISEASE COURSE
First-year clinical and brain MRI changes are independent predictors of conversion to MS and disability accumulation in patients with clinically isolated syndromes (CISs). Mar Tintoré, MD, Vall Hebron University Hospital, Barcelona, Spain, noted that baseline lesions and new T2 lesions during first year, as well as the use of disease-modifying therapy (DMT) before second attack, are independent predictors of further attacks. Oligoclonal bands (OCBs), new T2 lesions, and incomplete recovery are independent predictors of accumulation of disability.
In one large long-term CIS prospective study, baseline factors during the first year that predict conversion to clinically definite MS (CDMS) were examined. The baseline variables included demographic factors (age and sex), clinical signs (topography of CIS and onset date of DMT), presence of OCBs in cerebrospinal fluid (CSF), and brain MRI (number of T2 and Gd+ lesions). Outcomes were second attack occurring after 12 months or CDMS and EDSS ≥ 3.0 at 2 time points (disability accumulation). From 1995 to 2013, 1015 CIS patients underwent clinical and brain MRI follow-up (mean, 7.7 years). At baseline, a positive OCB was confirmed in 58.6% of patients; T2 lesions ≥ 10 were seen in 41.4%; and 22.8% had a presence of Gd+ lesions.
In the first year, a relapse occurred in 17.1% of patients; 23.6% had an EDSS ≥ 2.0; new T2 lesions ≥ 10 were seen in 6.6%; and Gd+ lesions on brain MRI occurred in 19% of patients.
Patients converting to CDMS had a significantly greater number of baseline and new T2 and Gd+ lesions when compared with patients not converting (P < .0001). New lesion formation was a positive predictor of a higher risk of CDMS and disability progression. EDSS score ≥ 3 was a positive predictor of new lesion formation, incomplete recovery, presence of OCBs, and treatment before second attack. Predicting the risk of a second MS attack can be improved by taking OCBs in the CSF and baseline/new T2 and Gd+ lesions into consideration.
INDEPENDENT PREDICTORS OF TIME TO RELAPSE AFTER CIS IN HIGH-RISK PATIENTS
Predicting risk factors that identify patients at higher risk of converting from first relapse post-CIS to CDMS can help establish optimal timing for initiating DMT. Tim Spelman, MD, University of Melbourne, Melbourne Brain Centre, Parkville, Australia, identified younger age at onset; no previous DMD exposure; the presence of T2 and Gd+ lesions or infratentorial, juxtacortical, or peroventricular lesions; and OCBs on baseline CSF as predictors of increased rate of conversion.
Patient (n = 3296) data obtained from the MS Base Incident Study subset of the MS Base global registry were used to analyze time to second attack based on a Cox poportional hazards regression [Butzkueven H et al. Mult Scler. 2006]. Demographic, treatment, and examintion characteristics were weighed to determine time to second attack post-CIS.
Increasing age at disease onset and lower EDSS score were associated with a reduced risk of relapse. At least 1 T2 or Gd+ lesion, at least 1 infratentorial and 1 juxtacortial lesion on cerebral MRI, and the presence of OCBs on baseline CSF were significant predictors of a second attack (P < .001; Table 1).
Predictors of First Relapse After Clinically Isolated Syndrome
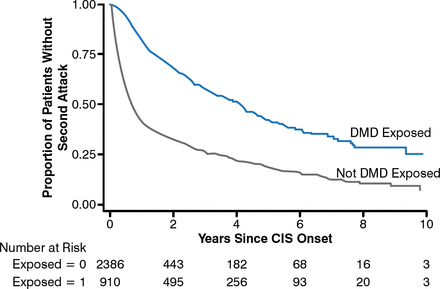
DMD exposure preconversion significantly increased the proportion of patients who did not experience a second attack (HR, 0.57; 95% CI, 0.46 to 0.72; P < .001; Figure 2).
Impact of DMD Exposure on Second Multiple Sclerosis Attack
CIS, clinically isolated syndrome; DMD, disease-modifying drug.
Reproduced with permission from T Spelman, MD.
Early identification of patients at higher risk of second MS attacks would allow clinicians to closely monitor MS patients and consider early treatment intervention, which may result in better patient outcomes.
- © 2014 MD Conference Express®