Summary
Four speakers presented information regarding updates in the management and treatment of atrial fibrillation, ventricular tachycardia ablation, and telemonitoring. Recently updated and forthcoming guidelines were presented related to anticoagulation in patients who undergo catheter ablation, tachycardia and syncope, management of patients with ventricular arrhythmias, and the prevention of sudden cardiac death.
- anticoagulation
- atrial fibrillation
- ventricular tachycardia
- catheter ablation
- cardiac resynchronization therapy
- telemonitoring
- smartphone
- anticoagulation
- weight management
Throughout 2015, there have been updates in recommendations, guidelines, and clinical evidence for the management of ventricular tachyarrhythmias, atrial fibrillation (AF), cardiac resynchronization therapy (CRT), and telemonitoring. Four speakers presented take-home messages regarding each of these areas.
Lucas Boersma, MD, St Antonius Hospital, Nieuwegein, The Netherlands, began the session with an update on AF guidelines. He announced that there will be a new European Society of Cardiology (ESC) guideline for AF published in 2016, and he reviewed the new guideline for antithrombotic management in patients undergoing catheter ablation. According to Prof Boersma, the ESC now recommends that 1) patients who undergo catheter ablation continue on uninterrupted anticoagulation with a vitamin K antagonist without heparin bridging, and 2) new oral anticoagulants should be interrupted for 24 hours prior to the procedure without heparin bridging [Sticherling C et al. Europace. 2015].
Prof Boersma then discussed lifestyle and weight management in patients at risk of developing AF. The LEGACY trial reported that long-term sustained weight loss was associated with a reduction of AF burden and maintenance of sinus rhythm [Pathak RK et al. J Am Coll Cardiol. 2015;65(20)]. In addition, cardiorespiratory fitness appears to augment the beneficial effects of weight loss in obese people who experience symptomatic AF [Pathak RK et al. J Am Coll Cardiol. 2015;66(9)].
AF is often asymptomatic and more prevalent in patients who receive a pacemaker or those who have experienced cryptogenic stroke or a transient ischemic attack [Sanna T et al. N Engl J Med. 2014]. He highlighted data from the PROTECT-AF [Reddy VY et al. JAMA. 2014] and the PREVAIL [Holmes DR et al. J Am Coll Cardiol. 2015] trials, which showed that percutaneous closure of the left atrial appendage in patients with nonvalvular AF was associated with improved risk of hemorrhagic stroke and all-cause mortality compared with vitamin K antagonists, and risk of stroke and embolism appeared to be at least noninferior.
Prof Boersma closed his presentation with several other take-home messages:
New ablation techniques are improving durable isolation of the pulmonary vein to increase freedom from paroxysmal AF.
Standardized substrate ablation beyond pulmonary vein isolation does not appear to improve freedom of persistent AF [Verma et al. N Engl J Med. 2015].
Detailed anatomic and electrical mapping must guide an individualized ablation strategy to cure AF.
Josef Kautzner, MD, Institute for Clinical and Experimental Medicine, Prague, Czech Republic, spoke about ventricular tachycardia (VT) ablation, about which there is still less published research. He highlighted recent studies on the use of enhanced cardiac magnetic resonance to visualize scar location in patients with structural heart disease and possibly corridors of conducting channels as a way of targeting VT [Fernández-Armenta J et al. Circ Arrhythm Electrophysiol. 2013]. The information generated from these scans improves our understanding of the substrate and has the potential to facilitate the ablation procedure by allowing the operator to direct the ablation catheter toward the target ablation site. Prof Kautzner then emphasized the role of the papillary muscles and/or the moderator band in triggering polymorphic VT or ventricular fibrillation. Intracardiac echocardiography provides excellent support in ablation of these triggering foci. Subsequently, he continued to discuss 2 main strategies of VT ablation in structural heart disease: either substrate mapping in sinus rhythm or during ventricular pacing or mapping in VT using mechanical support devices such as a percutaneous ventricular assist device (pVAD).
Prof Kautzner emphasized that in Europe, operators are less likely to use pVADs. He reviewed data showing no difference in clinical outcomes between the 2 strategies, but an apparent trend toward an increase in the percentage of complications in the pVAD group [Bunch TJ. Europace. 2011]. When comparing VT ablation using an intra-aortic balloon pump or a pVAD, again there were no significant differences in clinical outcomes. Complications were more frequent in the pVAD group, although the trend did not reach statistical significance (Table 1) [Reddy Y et al. Circ Arrythm Electrophysiol. 2014]. On the other hand, he underscored that catheter ablation can be successfully performed in patients with a left ventricular (LV) assist device who suffer from recurrent ventricular arrhythmias [Sacher F et al. Circ Arrhythm Electrophysiol. 2015].
Comparison of Baseline Characteristics Among Patients Undergoing VT Ablation With IABPs or Non-IABPs
Prof Kautzner then discussed the safety and efficacy of VT ablation in several different types of patients. In a study of 2061 patients with structural heart disease, 72% of patients with ischemic heart disease and 68% of patients with nonischemic cardiomyopathy were free from VT recurrence at 1 year [Tung R et al. Heart Rhythm. 2015]. Survival improved as the recurrence of VT decreased. There was no difference in the estimated rate of transplant or mortality. There appears to also be a role for VT ablation as the therapy of choice in patients with preserved ejection fraction after a myocardial infarction [Clemens M et al. J Cardiovasc Electrophysiol. 2015; Pauriah M et al. Circ Arrythm Electrophysiol. 2013].
Discussing the safety of VT ablation, Prof Kautzner reviewed data from 3 single centers suggesting that patients with structural heart disease had complication rates ranging from 6% to 8%, which included procedure-related mortality (0%-0.4%), tamponade (0.4%-2%), cerebrovascular accident (0%-0.8%), and vascular issues (2%-4.7%). An assessment of the safety of VT ablation in clinical practice reported that the incidence of adverse events associated with VT ablation is approximately 8.5% and it is 14.7% for patients with structural heart disease [Katz DF et al. Circ Arrythm Electrophysiol. 2015].
Haran Burri, MD, University Hospital of Geneva, Geneva, Switzerland, presented news in CRT and pacing. He reviewed 4 substudies from the original MADIT-CRT trial [Moss AJ et al. N Engl J Med. 2009] and summarized the findings as follows:
At a median 5.6 years of follow-up, findings indicated that early intervention with CRT was associated with a significant long-term survival benefit in patients with mild heart failure symptoms, LV dysfunction, and left bundle-branch block (LBBB) [Goldenberg I et al. N Engl J Med. 2014].
In patients without LBBB, those with a long PR interval derived a significant clinical benefit from the implantation of CRT vs an implantable cardioverter defibrillator (ICD) only [Kutyifa V et al. Circ Arrhythm Electrophysiol. 2014].
LV ejection fraction normalization was achieved in 7% of patients with CRT with a low rate of VTs. These patients could be considered for a downgrade to CRT-pacemaker only [Ruwald MH et al. Circulation. 2014].
Patients with ≥ 0.1% ectopic beats on a preimplantation 24-hour Holter monitoring have worse outcomes [Ruwald MH et al. J Am Coll Cardiol. 2014].
Prof Burri also reviewed data regarding quadripolar leads, which suggest that there is a high implant success rate [Crossley GH et al. Heart Rhythm. 2015] with few dislodgements and good thresholds [Sperzel J et al. Europace. 2015], as well as the elimination of phrenic nerve stimulation [Behar JM. J Cardiovasc Electrophysiol. 2015]. He also noted that PV loop-guided multipoint LV pacing resulted in greater LV reverse remodeling and increased LV function at 12 months compared with conventional CRT [Pappone C et al. Heart Rhythm. 2015].
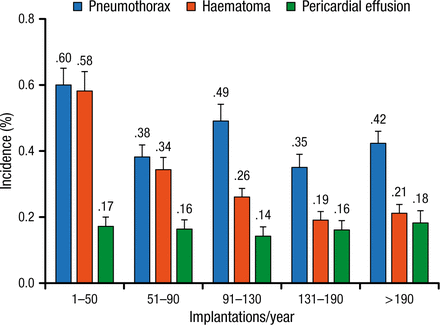
According to Prof Burri, an implant volume of < 50 devices per year is associated with a greater number of complications (Figure 1) [Nowak B et al. Europace. 2015]. He urged national societies to set standards for minimum operator volume in the interest of quality.
Rates of the 3 Most Frequent Surgical Complications According to Implantation Volume Quintiles
Reprinted from Nowak B et al, Association between hospital procedure volume and early complications after pacemaker implantation: results from a large, unselected, contemporary cohort of the German nationwide obligatory external quality assurance programme, Europace, Vol 17, issue 5, Pages 787-793, Copyright (2015), with permission from European Society of Cardiology.
Frieder Braunschweig, MD, Karolinska University Hospital, Stockholm, Sweden, focused on news regarding syncope and telemonitoring. He recommended that attendees read 3 recently published position papers: the 2015 Heart Rhythm Society Expert Consensus Statement on tachycardia and syncope [Sheldon RS et al. Heart Rhythm. 2015]; Syncope Unit: Rationale and Requirement, published by the European Heart Rhythm Association [Kenney RA et al. Europace. 2015]; and the 2015 ESC Guideline for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death [Priori SG et al. Eur Heart J. 2015].
Prof Braunschweig then discussed remote monitoring of pacemakers, defibrillators, and sensors. A meta-analysis of remote monitoring studies found that monitoring done either remotely or in the office showed comparable safety and survival overall outcomes, with a potential survival benefit seen in randomized trials that used daily transmission verification [Parthiban N et al. J Am Coll Cardiol. 2015]. Benefits of remote monitoring included more rapid clinical event detection and a significant reduction in inappropriate shocks (P = .002). He also highlighted results from the In-Time trial, which randomly assigned 664 patients with heart failure to telemonitoring or control [Hindricks G et al. Lancet. 2014]. Patients on telemonitoring had significantly lower modified Packer scores (P < .05) and rates of all-cause mortality (P = .004). According to Prof Braunschweig, < 75% of centers in Europe are using telemonitoring for patients with an ICD or a CRT, with physicians reporting a lack of reimbursement, technical issues, and increased workload as barriers to implementation [Mairese GH et al. Europace. 2015].
Telemonitoring can also be used creatively as a screening tool for patients who do not have an implanted device. Prof Braunschweig reviewed data from the STROKESTOP study, which used a portable device to record a thumb electrocardiogram over a 2-week period and transmitted it to a clinician via the Internet [Svennberg E et al. Circulation. 2015]. Among 7173 participants, 3% were found to have previously undiagnosed AF. In total, 5.1% of the screened cases were untreated; screening resulted in 3.7% of the patients agreeing to treatment with an oral anticoagulant.
Prof Braunschweig summarized his thoughts about smartphones as an opportunity to manage patients remotely and individualize care. However, he warned that there is currently no compelling evidence of clinical usefulness, that many apps provide uncertain or no added value, and that, in his opinion, quality standards and certifications are required.
- © 2015 SAGE Publications