Summary
The SUPPORT trial, involving 166 patients (mean age, 58 years) with acute myocardial infarction being treated with ticagrelor, demonstrated the value of an interactive smartphone app in boosting drug adherence and adopting/continuing healthy cardiovascular lifestyle changes. This and other disease-specific apps could be used to complement secondary prevention strategies.
- acute myocardial infarction
- adherence
- interactive app
- NCT01874262
- smartphone
- ticagrelor
A smartphone app that provides interactive feedback in response to patient input can be beneficial in motivating patients who have suffered a myocardial infarction (MI) to maintain or improve drug adherence and adopt/continue healthy cardiovascular lifestyle changes. The SUPPORT trial [NCT01874262], headed by Christoph Varenhorst, MD, PhD, Uppsala Clinical Research Center and Department of Medical Sciences, Cardiology, Uppsala University, Sweden, may herald the use of disease-specific apps to complement conventional secondary cardiovascular care.
In the SUPPORT trial, 166 patients (mean age, 58 years; 81% men; 21% current smokers) being treated with ticagrelor for acute MI were randomized to use the interactive smartphone app (active group, n = 86) or a simpler, noninteractive app that tracked only adherence to the ticagrelor regimen (control group, n = 80).
The study lasted about 6 months. Cardiovascular risk factors that were assessed at baseline, 6 to 10 weeks, and 23 to 25 weeks included body mass index, physical activity, blood pressure, low-density lipoprotein cholesterol, and smoking cessation. Quality of life, self-reported ticagrelor use, patient satisfaction, and safety were also assessed.
In the interactive version of the app, patient entry of data prompted visual feedback and messages that were intended to boost motivation concerning adherence to the ticagrelor regimen, to increase awareness of cardiovascular disease, and to enhance resolve to make and maintain healthy lifestyle changes in aspects including physical activity and quitting smoking (Figure 1).
Example Screen Views of the Interactive App
Reproduced with permission from C Varenhorst, MD.
At baseline, the active and control groups were similar in demographic and clinical characteristics (Table 1).
Patient Characteristics
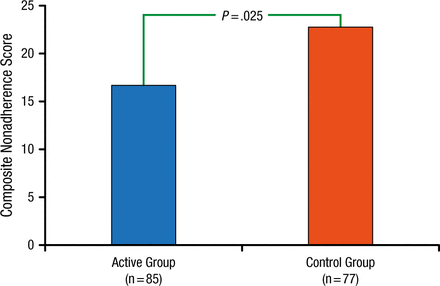
The primary end point was patient-registered drug nonadherence, defined as a composite end point of treatment failure (2 missed doses during a 7-day period) and a gap in treatment consisting of 4 consecutive missed doses. Self-reported adherence to the ticagrelor regimen at 6 months was significantly higher (P = .025) in the active group (16.6 ± 42.9 missed events) than in the control group (22.8 ± 41.3 missed events; Figure 2).
Primary End Point: Patient-Registered Drug Noncompliance
Reproduced with permission from C Varenhorst, MD.
Secondary analyses revealed a positive trend in the active group vs the control group for smoking cessation (80% vs 46%; P = .139), increased physical activity in terms of median change in weekly exercise minutes (90 vs 65; P = .612), and quality of life as measured with the EQ5D-VAS (14.7 vs 8.4; P = .059). Patients’ satisfaction was higher in the active group as compared with the control group (usability score of 100, 87 vs 78; P = .001).
The majority (68%) of active group participants would keep using the app if it were generally available, and most (97%) would recommend the app to other acute MI patients receiving drug therapy.
Among patients with acute MI, the interactive smartphone app was successful in enhancing motivation to continue drug therapy adherence and adopt healthy lifestyle changes. Thus, disease-specific apps could aid secondary prevention care.
- © 2015 SAGE Publications