Summary
A reservoir of risk in the treatment of dyslipidemia calls for optimization of statin treatment, which involves monitoring of response and compliance and adjustment of dose as needed. Also, other therapeutic approaches are needed. Niacin and fibrates are questionable options. Ezetimibe, PCSK9 inhibitors, mipomersen, and lomitapide have potential merit.
- dyslipidemia
- ezetimibe
- fibrates
- lomitapide
- mipomersen
- niacin
- PCSK9 inhibitors
- statins
While statins are the cornerstone for lipid-lowering therapy, many patients experience major cardiovascular (CV) events despite optimal statin therapy, thereby suggesting the need for additional options for lipid-lowering therapy. Marja-Riitta Taskinen, MD, PhD, Helsinki University Hospital, Helsinki, Finland, presented some alternatives for patients for whom statins might not be the best option.
Alternatives to statin in alleviating the risk of CV disease (CVD) include lowering of low-density lipoprotein cholesterol (LDL-C) using ezetimibe to block cholesterol absorption and using niacin or fibrates to raise the level of high-density lipoprotein cholesterol.
The future for niacin in dyslipidemia is questionable. While daily doses exceeding 1.5 g can reduce the risk of CVD events and progression of atherosclerosis, 2 large randomized clinical trials reported no clinical benefits [Boden WE et al. New Engl J Med. 2011; HPS2-THRIVE Collaborative Group. New Engl J Med. 2014]. Furthermore, niacin use has been associated with serious adverse effects [Landray M et al. New Engl J Med. 2014].
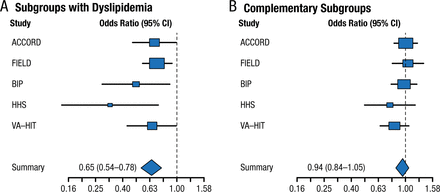
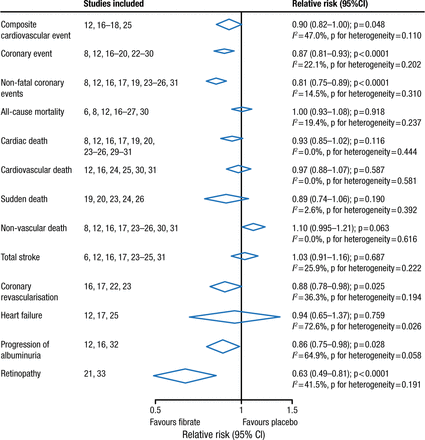
Fibrates have a long history in lowering triglycerides and raising high-density lipoprotein cholesterol. Two large randomized controlled trials involving > 15 000 patients with type 2 diabetes mellitus failed to demonstrate the clinical benefit of fenofibrate [ACCORD Study Group. New Engl J Med. 2010; Scott R et al. Diabetes Care. 2009; FIELD Study Investigators. Lancet. 2005]. However, meta-analyses, including the FIELD and ACCORD trials, reported a benefit in reduced CVD but not all-cause or CVD-related mortality among patients with vs without dyslipidemia (Figures 1 and 2) [Jun M et al. Lancet. 2010; Sacks FM et al. New Engl J Med. 2010]. Note that these results were driven by the VA-HIT and HHS trials, which did not use background intensive statin therapy.
Treatment Effect in Meta-Analysis of Fibrate Trials
From N Engl J Med, Sacks FM et al, Combination lipid therapy in type 2 diabetes, Vol 363, Pages 692-694, Copyright © (2010) Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Meta-analysis of Fibrates in 18 Trials
Reprinted from The Lancet, Vol 375, Jun M et al, Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis, Pages 1875-1884, Copyright (2010), with permission from Elsevier.
The benefit of ezetimibe added to statin therapy in > 5200 patients following acute coronary syndrome was examined in the IMPROVE-IT trial [Cannon CP et al. New Engl J Med. 2015]. An incremental lowering of LDL-C and improved CV outcomes were evident. The results indicate the value of drugs that lower LDL-C.
Current trials are addressing the inhibition of apolipoprotein B (apoB) as a means of regulating plasma triglyceride levels and remnant cholesterol and the use of n-3 fatty acids in patients with elevated triglycerides.
Future Drug Development for Dyslipidemia
Lessons learned in the development of mipomersen and lomitapide are instructive for future drug development in dyslipidemia, according to John Kastelein, MD, PhD, University of Amsterdam, Amsterdam, The Netherlands.
Mipomersen is an antisense nucleotide that is subcutaneously injected to inhibit the production of apoB in the liver. Mipomersen is the latest in a long line of drugs targeting apoB. Success in lowering LDL-C via apoB inhibition has been elusive. Randomized double-blind placebo-controlled trials have demonstrated the efficacy and tolerability of mipomersen in reducing LDL-C in homo- and heterozygous familial hypercholesterolemia (Table 1) [Raal FJ et al. Lancet. 2010; McGowan MP et al. PLoS ONE. 2012; Stein EA et al. Circulation. 2012].
Data From Phase 3 Trials of MIPO
Lomitapide inhibits microsomal triglyceride transfer protein in the liver and intestine, which decreases secretion of very-LDL-C into the bloodstream in clinical trial cohorts [Cuchel M et al. Lancet. 2013; Cuchel M et al. New Engl J Med. 2007] and individuals [Rader DJ, Kastelein JJ. Circulation. 2014]. Adverse effects are mainly gastrointestinal and tend to wane with time, with no evidence of liver damage.
Both drugs have promise and may herald the subsequent development of apoB-targeted therapy.
- © 2015 SAGE Publications