Summary
This article highlights select advances in the intensive management of low-density lipoprotein cholesterol levels. It discusses the use of statins as the cornerstone of treatment, addresses the use of novel adjuvant therapies for reducing atherogenic lipoproteins, and highlights data from important new studies demonstrating the safety and clinical impact of much more aggressive low-density lipoprotein cholesterol reduction in patients with established coronary artery disease.
- cardiovascular disease risk
- DYSIS
- IMPROVE-IT
- low-density lipoprotein cholesterol
- ODYSSEY
- OSLER
- cardiology & cardiovascular medicine guidelines
- lipid disorders
- cardiology & cardiovascular medicine clinical trials
A panel of speakers shared highlights of recent advances in the management of low-density lipoprotein cholesterol (LDL-C) in patients at high risk of future cardiovascular (CV) events.
State of the Art in LDL Lowering
According to Peter P. Toth, MD, PhD, CGH Medical Center, Sterling, Illinois, USA, statins remain the primary pharmacologic treatment option for reducing LDL-C levels. Studies have consistently shown that statin-induced LDL-C reductions decrease CV events in primary and secondary populations. Data from a patient-level meta-analysis of statin trials demonstrated that the lowest level of achieved LDL-C (≤ 50 mg/dL [1.3 mmol/L]) is associated with the lowest risk for CV events [Boekholdt SM et al. J Am Coll Cardiol. 2014]. The IMPROVE-IT trial demonstrated reduced CV risk with achievement of a mean LDL-C of 54 mg/dL (1.4 mmol/L) in the simvastatin/ezetimibe arm compared with a mean of 69 mg/dL (1.8 mmol/L) in the simvastatin arm [Cannon CP et al. N Engl J Med. 2015]. These studies, among others, provide evidence for the benefit of lowering LDL-C beyond prior LDL-C treatment goals for the first time with a lipid-lowering therapy beyond statins alone.
Dr Toth noted that patients with primary dyslipidemia and the heterozygous form of familial hypercholesterolemia (FH) requiring adjuvant therapy for incremental LDL-C reduction can be treated with ezetimibe, as well as proprotein convertase subtilisin/kexin 9 (PCSK9) monoclonal antibodies, including alirocumab [Kastelein JJ et al. Eur Heart J. 2015] and evolocumab [Gouni-Berthold I, Berthold HK. Curr Pharm Des. 2014]. Emerging data suggest that LDL-C reduction through monoclonal antibody inhibition of PCSK9 may reduce CV risk [Robinson JG et al. N Engl J Med. 2015; Sabatine MS et al. N Engl J Med. 2015].
In the OSLER trial, evolocumab therapy led to a 61% relative reduction (P < .0001) and a 73 mg/dL (1.9 mmol/L) absolute reduction of LDL-C beyond that achieved by background statin and other lipid-lowering therapies. This was associated with a 53% relative risk reduction (P = .003) of an expanded CV end point (death, myocardial infarction, hospitalization for unstable angina or heart failure, coronary revascularization, stroke, or transient ischemic attack) at 1 year of therapy [Sabatine MS et al. N Engl J Med. 2015]. In the ODYSSEY long-term trial, alirocumab therapy led to similar LDL-C reductions and a 48% relative risk reduction in CV events at 18 months of follow-up [Robinson JG et al. N Engl J Med. 2015]. Outcomes trials for both drugs are underway. To date, the safety and tolerability data for both drugs have been encouraging.
Among patients with the homozygous form of FH (most often from deleterious low-density lipoprotein [LDL] receptor mutations), Dr Toth discussed the use of lomitapide [Cuchel M et al. Lancet. 2013] and mipomersen [Santos RD et al. Eur Heart J. 2015; Goldberg AC. J Clin Lipidol. 2010] as novel approaches to reduce LDL-C in patients with poorly or nonfunctioning LDL receptors. Lomitapide is an oral microsomal triglyceride transfer protein inhibitor that reduces the production and secretion of very low-density lipoprotein (VLDL) from the liver. This results in fewer circulating VLDL particles that can undergo metabolism into LDL-C. Mipomersen is an injectable antisense oligonucleotide that binds to the mRNA of apoprotein B100, the primary apoprotein constituent of VLDL. Apoprotein B100 production is inhibited, which results in a significant reduction in VLDL production and secretion (with subsequent reductions in LDL-C). Both agents can increase hepatic steatosis, but this effect is reversible, Dr Toth noted.
The Safety and Efficacy of Very Low LDL-C Levels
Data from the IMPROVE-IT study in post–acute coronary syndrome (ACS) patients showed that intensive combination therapy with ezetimibe and simvastatin, compared with simvastatin alone, led to further reduction in achieved LDL-C levels of 54 vs 69 mg/dL (1.4 vs 1.8 mmol/L), and a significant 2% absolute reduction in CV events without an increase in the rates of adverse events (AEs) [Cannon CP et al. N Engl J Med. 2015]. Because of these low achieved LDL-C levels and a long follow-up (average 6 years) of > 18 000 patients, the IMPROVE-IT data allowed for evaluation of whether very low achieved LDL-C might be associated with safety or tolerability concerns, said Robert P. Giugliano, MD, SM, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA.
Dr Giugliano shared data from a post hoc analysis that pooled both arms of the IMPROVE-IT trial and then stratified patients into the following 4 groups according to achieved LDL-C levels at 1 month: 6% of patients were in the < 30 mg/dL (0.8 mmol/L) group; 31% were in the 30 to < 50 mg/dL (0.8 to < 1.3 mmol/L) group; 36% were in the 50 to < 70 mg/dL (1.3 to < 1.8 mmol/L) group; and 26% were in the ≥ 70 mg/dL (1.8 mmol/L) group. Safety and efficacy outcomes were then compared among the 4 groups.
Overall, the data showed that the safety profile in post-ACS patients with a very low LDL-C (< 30 mg/dL [0.8 mmol/L]) level was similar to those with higher achieved LDL-C levels over prolonged follow-up. Patients with lower LDL-C levels were more likely to have been assigned to the ezetimibe and simvastatin arm. Trends across the 4 groups demonstrated that patients with lower achieved LDL-C were also more likely to be older, men, and statin naïve, with a prior history of diabetes and hypertension; however, they were less likely to have had a prior myocardial infarction or history of coronary revascularization, or be a current smoker, than those with higher levels.
Compared with the other 3 groups, patients who achieved LDL-C levels < 30 mg/dL (0.8 mmol/L) did not experience an increased rate of AEs, including AEs leading to discontinuation of lipid-lowering therapy (P = .93), abnormalities in liver function tests (P = .28), muscle-related events (P = .74), cognitive impairment (P = .37), or hemorrhagic stroke (P = .42).
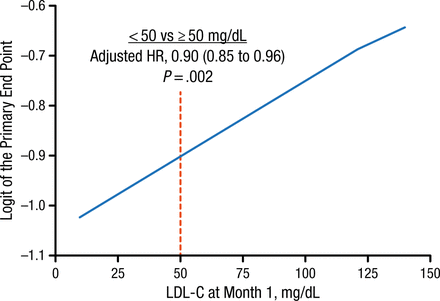
Efficacy data showed that patients who maintained lower LDL-C levels had a decreased probability of experiencing a CV event. Achievement at 1 month of an LDL-C level < 50 mg/dL (< 1.3 mmol/L), compared with ≥ 50 mg/dL (≥ 1.3 mmol/L), was associated with a 10% reduction in the composite primary end point of CV death, nonfatal myocardial infarction, unstable angina requiring rehospitalization, coronary revascularization (≥ 30 days after randomization), or nonfatal stroke (Figure 1).
Risk of Cardiovascular Events at Different LDL-C Levels
LDL-C, low-density lipoprotein cholesterol.
Reproduced with permission from RP Giugliano, MD.
Similarly, when data were analyzed by quartiles of LDL-C achieved at 4 months, there was no significant difference in AEs across the quartiles (< 45 mg/dL [< 1.2 mmol/L]; 45 to < 60 mg/dL [1.2 to < 1.6 mmol/L]; 60 to < 75 mg/dL [1.6 to < 1.9 mmol/L]; ≥ 75 mg/dL [≥ 1.9 mmol/L]). In addition, there was greater reduction in CV events in the quartile with an achieved LDL-C level < 45 mg/dL (< 1.2 mmol/L) compared with that in which levels were ≥ 75 mg/dL (≥ 1.9 mmol/L; HR, 0.91; 95% CI, 0.85 to 0.98).
These findings therefore support continuation of intensive lipid-lowering therapy without modification in patients achieving very low LDL levels, said Dr Giugliano.
DYSIS: Comparison of Results From Statin-Treated Patients in Europe and China
Anselm Kai Gitt, MD, Herzzentrum Ludwigshafen, Germany, discussed results of the DYSIS Registry. This cross-sectional, observational study aimed to investigate lipid goal attainment among statin-treated patients with very high CV risk, defined according to the 2011 European Atherosclerosis Society and European Society of Cardiology guidelines for the management of dyslipidemia [Reiner Z et al. Eur Heart J. 2011]. In this global collaboration, data were collected in physicians’ offices and hospital outpatient wards between 2008 and 2012 in Canada, Europe, Middle East countries, and China.
The study included patients aged ≥ 45 years who were currently receiving statin treatment and who had a documented fasting lipid profile that was performed within the past 12 months, following at least 4 weeks of statin therapy, and contained ≥ 1 lipid parameter.
Dr Gitt shared data comparing the level of LDL-C goal achievement in Europe and China. Of the 57 090 patients receiving chronic statin therapy, 25 317 were enrolled in China and 31 773 in Europe. Dr Gitt highlighted some major differences in the CV risk profiles among patients in the 2 regions. Chinese patients were strikingly less likely to have first-degree relatives with coronary disease than Europeans (9.1% vs 30.2%). Chinese patients were also less likely to be smokers (12.4% vs 14.5%), have a sedentary lifestyle (19.7% vs 50.0%), be obese (5.5% vs 34.9%), or have diabetes (34.7% vs 37.8%) or hypertension (65.8% vs 77.1%; P < .01 for all).
Patients in China were also significantly less likely to have suffered ischemic heart disease (37.2% vs 40.8%), heart failure (3.8% vs 12.2%), or peripheral artery disease (1.0% vs 9.7%; P < .01 for all). However, Chinese patients were significantly more likely than European patients to have suffered cerebrovascular disease (16.9% vs 9.9%; P < .01).
Although statins comprised the cornerstone of therapy in both regions, patients in China received lower median doses compared with those in Europe (20.1 vs 27.6 mg per day simvastatin; 17.5 vs 25.8 mg per day atorvastatin; 9.7 vs 12.9 mg per day rosuvastatin).
Nevertheless, in both regions, only one-third of patients reached the recommended LDL-C target < 70 mg/dL (< 1.8 mmol/L). In addition, Chinese patients were significantly more likely to have combined dyslipidemia than patients in Europe (39% vs 32.1%; P < .01), triglyceride levels > 150 mg/dL (> 1.7 mmol/L; 42.6% vs 38.2%; P < .01), and high-density lipoprotein cholesterol levels < 40/45 mg/dL in men/women (< 1/1.2 mmol/L; 33.2% vs 26.9%; P < .01)
These data emphasize the need for increased focus on the use of lipid-lowering therapy to attain target LDL-C levels and therefore improve CV risk in both China and Europe, concluded Dr Gitt.
LDL-C–Lowering Strategies: Which Is Best?
According to Fernando H. Cesena, MD, Hospital Israelita Albert Einstein, São Paulo, Brazil, treatment of hypercholesterolemia is traditionally based on LDL-C goals, with a typically recommended goal of < 70 mg/dL (< 1.8 mmol/L) for high-risk patients. However, the 2013 American Heart Association (AHA)/American College of Cardiology (ACC) guidelines changed this concept, and instead now recommend moderate- to high-intensity statins to lower LDL-C levels by ≥ 30% in high-risk patients, making no recommendation for a specific LDL-C treatment goal.
Prof Cesena presented data from a simulation study that aimed to compare the estimated impact of LDL-C percent reduction vs goal strategies on the risk of major CV events in a real-world, single-institution, primary prevention population (n = 1716) with a predicted 10-year risk for atherosclerotic CV disease ≥ 7.5% according to the AHA/ACC pooled risk equation. Researchers simulated the impact of 4 treatment strategies: 2 based on LDL-C percent reduction (30% [S30%] or 50% [S50%]), and 2 based on achievement of goal LDL-C level (≤ 70 mg/dL [S70] or ≤ 100 mg/dL [S100]).
Although all strategies would reduce both mean LDL-C levels and CV risk from baseline, the more aggressive strategies would perform significantly better: S50% would be more effective than S30%; S70 would be more effective than S100 (P < .001 for all). Both aggressive strategies could potentially prevent 50% more major CV events than their less aggressive counterparts.
According to Prof Cesena, the results showed that a strategy based on a 50% LDL-C reduction would avoid a similar number of major CV events as one that aims to achieve a target LDL-C level ≤ 70 mg/dL (47 events prevented per 1000 individuals, for both strategies). However, he emphasized that the best strategy for each individual would likely depend on the baseline LDL-C level. In the simulation, about one-half of patients simulated to achieve goal by one aggressive strategy would also successfully achieve the goal of the other aggressive strategy (eg, < 70 mg/dL or 50% reduction).
- © 2015 SAGE Publications