Summary
This randomized trial was designed to compare the efficacy of venlafaxine with supportive management with venlafaxine with problem-solving therapy in older patients with comorbid chronic low back pain and depression. Although both treatment options improved pain and depression scores, problem-solving therapy provided no additional benefit.
- venlafaxine
- supportive management
- problem-solving therapy
- chronic low back pain
- depression
- mood disorders
- psychiatry & psychology clinical trials
Jordan F. Karp, MD, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA, presented data from the ADAPT trial [NCT01124188], which demonstrated that venlafaxine with supportive management (SM) led to significant response rates, especially for pain, in older adults with depression and chronic low back pain (CLBP). However, there was no additional benefit of problem-solving therapy for depression and pain (PST-DP) in terms of improved response.
According to Dr Karp, late-life depression is a significant public health problem that decreases quality of life and survival in affected patients, contributes to a poorer prognosis for comorbid conditions, and is a risk factor for suicide. In addition, it is associated with increased health care utilization and costs. He noted that, when treating older adults, late-life treatment-resistant depression is the rule, not the exception. In the United States and Canada, 25% to 50% of older adults in community settings and 49% to 83% in nursing homes suffer chronic pain, reported Dr Karp. Anxiety and depression are also more common in these patients than in those without pain, and the pain can lead to memory and cognition problems. CLBP in particular has a prevalence of 12% in the community, and it is the most common referral to pain clinics.
The ADAPT study was therefore conducted to compare high-dose venlafaxine with PST-DP with high-dose venlafaxine with SM in older adults living with CLBP and depression. Inclusion criteria were men and women aged > 60 years experiencing CLBP and low mood. Primary outcomes were measures of depression, pain, and disability.
Two hundred and twenty-seven patients with comorbid depression and CLBP started the trial. In the first phase, all participants received 150 mg/d of venlafaxine for 6 weeks and SM. Phase 1 nonresponders had a higher medical burden than responders, said Dr Karp. They had more severe depression (Patient Health Questionnaire-9 [PHQ-9] score 16.5 vs 14.3; P = .004), more treatment-resistant depression (as measured by the Antidepressant Treatment History Form; 55.3% vs 18.4%; P = .0002), greater pain extensity (as demonstrated by the number of painful areas on a pain map; 13.5 vs 9.2; P = .01), and more pain-related functional disability (Roland Morris Disability Questionnaire score 15.61 vs 12.87; P = .0007). In phase 1, a 2-week change in the numeric rating scale was the only significant predictor of improvement in depression and pain.
Patients who responded poorly during phase 1 went on to the second intervention phase, while those who responded well were excluded. Patients received up to 300 mg/d (median dose 244 mg) of venlafaxine for 14 weeks and were randomized to also receive either SM or PST-DP (an average of 8 to 9 sessions). Response during phase 2 was characterized by 2 sequential visits of PHQ-9 ≤ 5 and ≥ 30% reduction in the numeric rating scale.
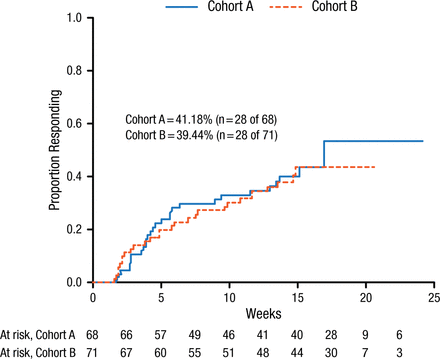
The results in phase 2 demonstrated a 40% response rate in depression and pain at any point during the study (Figure 1).
Response Rate in Phase 2 of the ADAPT Study
Cohorts A and B represent the 2 cohorts in the second phase of the study (cohorts remain blinded at this time).
Reproduced with permission from JF Karp, MD.
Although there was no additional benefit of PST-DP in terms of improved response, patient follow-up will continue for 12 months to investigate whether PST-DP decreases the rate of relapse and health care utilization, Dr Karp concluded.
- © 2015 SAGE Publications