Summary
Florian Holsboer, director of the Max-Planck Institute for Psychiatry for 25 years, called for a reengineering of the drug development process for antidepressants, meaning a shift from a focus on blockbuster drugs capable of treating all depression to use of biomarkers to target subsets of patients based on disease etiology.
- antidepressant
- depression
- monoaminergic
- imipramine
- CRH
- vasopressin
- ACTH
- hypothalamus
- pituitary
- HPA axis
- CRH receptor antagonist
- psychiatry & psychology clinical trials
- mood disorders
Florian Holsboer, MD, PhD, HMNC Brain Health, Munich, Germany, said that new approaches must be taken for the development of antidepressant drugs. Dr Holsboer said that the pharmaceutical industry’s fixation on blockbuster drugs mandates that an antidepressant drug candidate be one that works generally on all depression. However, it is becoming clear that depression is caused by many different mechanisms, which requires stratification of the population of patients with depression and opens the door to personalized medicine for depression.
Briefly outlining the history of antidepressant therapy, Dr Holsboer explained how the current paradigm became established, beginning with the discovery that imipramine had antidepressant properties, even though it was created in an attempt to mimic the activity of chlorpromazine. The success of imipramine led to development of a series of other, similar monoaminergic agents. Prescription of these drugs also led to dramatic decreases in suicide rates in Western Europe and North America, as well as revenue increases for pharma companies, with a considerable rise in antidepressant prescriptions between 2001 and 2011.
However, despite increased usage, entry of generic antidepressants into the market has decreased profits, and big pharma is abandoning development of new antidepressants, based partially on some notable phase 3 failures of alternative nonmonoaminergic agents. Dr Holsboer cited insufficiently predictive preclinical (animal) studies as contributing to the failures but also said that some drugs that were late development failures did not necessarily have negative results: The design of the trial, in placing the candidate against a monoaminergic comparator, was itself the source of failure.
Current antidepressants, monoaminergic compounds that are all relatively similar, work via enhancement of serotonin, noradrenaline, and dopamine neurotransmission. When given to the right patients at the right dose, these established therapies can result in 60% to 70% remission, said Dr Holsboer, but this leaves 30% to 40% of patients untreated, with side effects being yet another downside to these agents. The untreated minority is evidence that depression is caused by multiple mechanisms. Therefore, it is important to continue to address subpopulations in depression that are not helped by the available therapies, by continuing to search beyond monoaminergic agents.
In terms of directing the antidepressant drug development process to seek alternate targets for antidepressant drugs, Dr Holsboer cited the stress hormone system—driven by 2 neuropeptides, corticotrophin-releasing hormone (CRH) and vasopressin—as perhaps one of the most promising areas. Vasopressin works with CRH released by the hypothalamus in response to stress, to drive secretion of adrenocorticotropic hormone (corticotrophin) from the pituitary, which then causes the adrenal glands to secrete the stress hormone cortisol to create the stress response in the body and brain. Vasopressin and CRH regulate not only this hypothalamic-pituitary-adrenal axis but also behavioral adaptations to stress.
Depression is often a stress-induced disease, with symptoms of stress, such as loss of appetite and sleep disorders, leading to increased risk of depression; many patients with depression show stress abnormalities, such as hypercortisolism. Not everyone exposed to chronic stress develops depression; instead, this seems to occur in a subset of individuals at enhanced risk for stress-induced depression [Holsboer F et al. Ann Rev Psychol. 2010]. This subset is the target population of patients with depression for antidepressants based on stress hormone therapy. Although only a portion of the overall depressed population can be helped by a drug like this, Dr Holsboer said that this is in fact the point: The subgroup will benefit greatly and specifically with the therapy. The controlled randomized trials for such a drug should be conducted only on individuals from the subgroup, to avoid the situation where the comparator of general use will always be superior in the general group. The difficulty is determining in advance which patients are likely to benefit from the treatment.
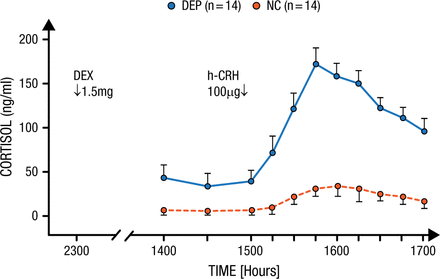
Dr Holsboer described a test that he and a colleague developed over 25 years ago to determine differential sensitivity to CRH in a population of patients with depression [Bardeleben U, Holsboer F. J Neuroendocrinol. 1989]. Patients were pretreated with dexamethasone, a powerful glucocorticoid, 1 day before an infusion of 100 µg of CRH to measure how much cortisol would be released. Normal controls showed only a modest increase in cortisol in response to CRH infusion because of the pretreatment, while patients with depression particularly sensitive to CRH had markedly elevated cortisol from the infusion (P < .01; Figure 1).
Increased Stress Hormone Released in Patients With Depression
In normal controls (NC), who were pretreated the day before with 1.5mg dexamethasone, an infusion of 100 µg hCRH induced only a modest elevation of plasma cortisol. In contrast, depressed patients (DEP) responded with a significantly increased cortisol secretion.
Reprinted from von Bardeleben U et al. Cortisol Response to a Combined Dexamethasone-Human Corticotrophin-Releasing Hormone Challenge in Patients with Depression. J Neuroendocrinol. 1989; 1: 485-488. Copyright (1989) Oxford University Press.
After describing the CRH sensitivity test, Dr Holsboer explained how such stratification tools could be used to economize the development process. Currently, clinical trials constitute >80% of development costs because when patients in trials have many different causes for their depression, researchers must recruit a very large number of patients to demonstrate a discriminatory effect. Describing personalized medicine as a departure from statistical medicine, Dr Holsboer said that such studies can be much smaller and therefore cheaper. The most important requirement of a reengineered drug development process is to identify ineffectiveness of a drug candidate as soon as possible. If 90% of drug candidates are destined to fail, then benefit is found in a quick failure, freeing resources for other candidates. One important step in that process is to move more quickly to humans and avoid extensive animal experiments.
- © 2015 SAGE Publications