Summary
This article discusses promising strategies in the management of treatment-resistant major depressive disorder. These include the use of pharmacologic approaches to modulate the hypothalamic-pituitary-adrenal axis, glutamatergic system, and endogenous opioid system, as well as nonpharmacologic approaches such as deep brain stimulation.
- major depressive disorder
- hypothalamic-pituitary-adrenal axis
- glutamatergic system
- endogenous opioid system
- deep brain stimulation
- mifepristone
- ketamine
- botulinum toxin
- pharmacogenetics
- psychiatry & psychology clinical trials
Alan F. Schatzberg, MD, Stanford University School of Medicine, Stanford, California, USA, provided an update on antidepressant treatment, highlighting new areas of antidepressant development, and discussed the potential use of pharmacogenetics to treat major depressive disorder (MDD).
According to Dr Schatzberg, although emergence of new antidepressants has been lacking in recent years, some progress has been made. He discussed some strategies that have been used in the search for new antidepressant treatments.
Targeting the Hypothalamic-Pituitary-Adrenal Axis
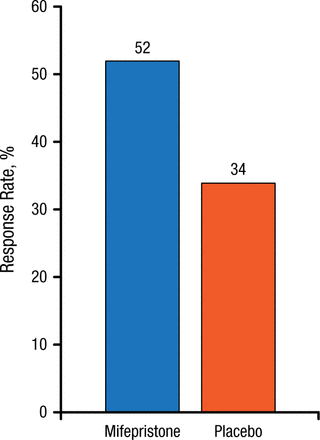
Individuals with psychotic major depression (PMD) comprise a subset of approximately 15% to 19% of patients with MDD who also have psychoses or delusions. However, the diagnosis of PMD is frequently overlooked, typically because patients are unwilling to discuss their psychotic symptoms, and even when they do, clinicians can fail to recognize their significance. Dr Schatzberg explained that these psychotic episodes are characterized by excessive hypothalamic-pituitary-adrenal axis activity, and affected patients have very high levels of cortisol. This has led to the hypothesis that antagonizing the cortisol response could lead to symptom resolution, and the glucocorticoid receptor antagonist mifepristone has shown efficacy in PMD. In one study, at plasma concentrations > 1660 ng/mL, mifepristone produced a rapid reduction in psychotic symptoms [Blasey CM et al. J Clin Psychopharmacol. 2011]. The response criterion was that patients experienced a 50% reduction in psychotic symptoms by day 7 compared with placebo, and this effect needed to be sustained to day 56 (Figure 1). Mifepristone remains in phase 3 studies in this area.
Efficacy of Mifepristone (Plasma Concentration > 1660 ng/mL) in Psychotic Major Depression
P = .023.
Source: Blasey CM et al. J Clin Psychopharmacol. 2011.
Reproduced with permission from AF Schatzberg, MD.
Bipolar depression also appears to be associated with elevated cortisol activity. In one study, mifepristone treatment produced some improvement in spatial working memory by day 14, and the benefit was much larger and attained statistical significance at 56 days [Watson S et al. Biol Psychiatry. 2012].
Targeting the Glutamatergic System
Glutamate is a key neurotransmitter in the central nervous system; the NMDA (N-methyl-D-aspartate) receptor (NMDAR) is a post-synaptic glutamate receptor, and some NMDA antagonists that block glutamatergic binding to NMDAR have demonstrated antidepressant-like effects in humans and animal models.
There has been much recent interest in the use of the anesthetic ketamine as an antidepressant. However, although intravenous administration of this NMDA antagonist produced immediate antidepressive effects, its main drawback lies in its lack of sustained effect beyond just a few days in most patients [Murrough JW et al. Am J Psychiatry. 2013]. Although other NMDA antagonists, such as memantine, have been investigated [Lenze EJ et al. Int J Geriatr Psychiatry. 2012], they have not managed to mimic the beneficial effects of ketamine.
Additional approaches have also been used, including the use of AZD6765, which, instead of blocking the NMDAR, acts to trap synaptic glutamate. In one study, AZD6765 improved depressive symptoms within 80 minutes, but the effect was not sustained beyond 110 minutes [Zarate CA Jr et al. Biol Psychiatry. 2013]. Recent trials have also failed to demonstrate its efficacy, using protocols involving multiple doses per week over several weeks, and the program has now been canceled.
Other drugs act as agonists or partial agonists at the postsynaptic receptor. GLYX-13, for example, acts as a partial NMDAR agonist and demonstrated antidepressive effects in rat models, without the unwanted ketamine-like side effects (SEs) [Burgdorf J et al. Neuropsychopharmacology. 2013].
D-cycloserine (DCS), an antibiotic and partial NMDAR antagonist that functions as an agonist at high doses, has also been evaluated, and Dr Schatzberg highlighted data from a recent study that demonstrated the benefit of add-on high-dose DCS in treatment-refractory patients with MDD [Heresco-Levy U et al. Int J Neuropsychopharmacol. 2013].
Targeting the Endogenous Opioid System
The endogenous opioid system is another potential therapeutic target for depression. Dr Schatzberg discussed a study by Koran and colleagues in 2005, in which oral morphine was significantly more effective than placebo in refractory obsessive-compulsive disorder. Beneficial effects were seen the day after treatment and lasted for 5 days.
He noted that the mechanism of action of ketamine as an antidepressant might involve more than just NMDAR blockade. One recent study demonstrated the involvement of endogenous opioids and µ- and δ-opioids in the central antinociception produced by ketamine [Pacheco DF et al. Brain Research. 2014]. Dr Schatzberg therefore suggested that, in addition to NMDAR antagonism, ketamine might also be acting as an opioid to produce its antidepressant effect.
Botulinum Toxin Treatment
Two double-blind studies have reported that a single treatment of the glabellar region with botulinum toxin produced a relatively rapid and sustained improvement of MDD symptoms in treatment-refractory patients [Finzi E, Rosenthal NE. J Psychiatr Res. 2014; Wollmer MA et al. J Psychiatr Res. 2012].
Pharmacogenetics
Pharmacogenetics are being adopted in the treatment of depression, and Dr Schatzberg noted that to date, SEs tend to be more readily predicted than are positive responses to specific agents. In addition, pharmacodynamic contributions appear to be more important than pharmacokinetics with respect to selective serotonin reuptake inhibitor (SSRI) SEs. He shared data from older studies showing that, although the cytochrome P450 2D6 (CYP2D6) genotype was not a significant concern during monotherapy with mirtazapine or paroxetine, variants in the short form of the serotonin transporter promoter polymorphism (5HTTLPR; S allele) influenced treatment response. In particular, the S allele was associated with SSRI intolerability: 50% of patients receiving paroxetine had dropped out of the study by 7 weeks, due to SEs. Recent commercial efforts are using an algorithm to assess genetic testing results of both pharmacokinetic and pharmacodynamic markers to guide treatment selection. Large scale trials are underway.
Deep Brain Stimulation
Development of nonpharmacologic methods to treat depression have also made advances over recent years. Dr Schatzberg stated that several case series, and ultimately 2 clinical trials, have investigated deep brain stimulation in patients with depression. Placement at the 2 locations in the recent blinded trials (subgenual cortex and anterior capsule) failed to produce significant benefit compared with sham treatment. A pilot trial suggested that stimulation of the medial forebrain bundle might be a preferred site, resulting in immediate improvement of symptoms in patients with treatment-refractory MDD [Schlaepfer TE et al. Biol Psychiatry. 2013].
Dr Schatzberg noted that the lack of significant advances in medication for depression in recent decades has resulted in decreased research efforts in this field by the pharmaceutical industry. The increasing placebo response rate in antidepressant trials and meta-analyses has been a significant problem in drug development, and he emphasized that although better end points are needed in antidepressant trials, specific markers remain lacking. Consequently, until better end points are identified in antidepressant trials, antidepressant drug development will continue to be problematic. Ketamine therefore represents an exciting innovation in the development of novel antidepressants, but additional work is needed to determine the precise mechanism of action involved in its antidepressant effect, concluded Dr Schatzberg.
- © 2015 SAGE Publications