Summary
The first-ever placebo-controlled trial in major depressive disorder with mixed features demonstrated that lurasidone, an atypical antipsychotic agent, was efficacious in reducing depressive symptoms in these patients and was also well tolerated when compared with placebo.
- major depressive disorder with mixed features
- lurasidone
- atypical antipsychotic
- MADRS total score
- CGI-S score
- psychiatry & psychology clinical trials
Trisha Suppes, MD, PhD, Stanford University School of Medicine, Stanford, California, USA, presented the results of a randomized controlled trial in adults with major depressive disorder (MDD) with mixed features, demonstrating that lurasidone significantly reduced depressive symptoms in these patients when compared with placebo [RESOLVE2; NCT01423240].
According to the DSM-V, the specifier “with mixed features” can be added to a diagnosis of MDD to indicate symptoms of both depression and subthreshold mania or hypomania. Although mixed features are present in 20% to 40% of individuals with MDD, this patient population is challenging to manage because of a lack of established treatments for this disease subset, Dr Suppes said. In addition to its poor treatment response, MDD with mixed features is associated with greater symptom severity and increased suicide risk. Evidence also suggests that this disease subset may be best classified within the spectrum of bipolar disorders [Vieta E, Valentí M. J Affect Disord. 2013].
Consequently, this multinational double-blind study was designed to investigate the efficacy and safety of lurasidone in patients with unipolar MDD with mixed (subthreshold hypomanic) features. Lurasidone is an atypical antipsychotic with efficacy in the treatment of bipolar depression, as both monotherapy and adjunctive therapy [Loebel A et al. Am J Psychiatry. 2014a, 2014b].
Inclusion criteria included patients who met the criteria for MDD according to the DSM-IV-TR, who had a Montgomery-Asberg Depression Rating Scale (MADRS) score ≥ 26, and who were experiencing 2 or 3 manic symptoms per the DSM-V criteria on most days over at least the 2 weeks prior to screening. Exclusion criteria included any lifetime history of bipolar I manic episodes or any mixed manic episodes.
Two hundred and eleven study participants were randomly assigned to 6 weeks of treatment with either flexible doses of lurasidone (20 to 60 mg/d; n = 109) or placebo (n = 100). The primary end point was the change in MADRS total score from baseline to 6 weeks, and the key secondary end point was the change in Clinical Global Impression–Severity score from baseline to 6 weeks. Responder rates were characterized as ≥ 50% reduction from baseline in MADRS total score.
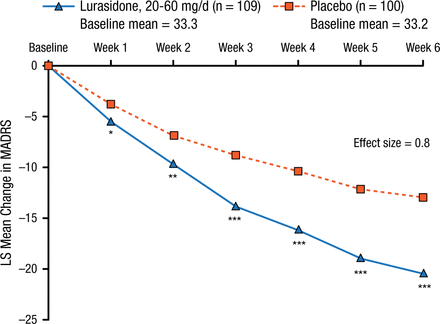
Lurasidone treatment was associated with a significantly greater mean reduction in MADRS total score (20.5 vs 13.0; P < .001; effect size, 0.80; Figure 1) and Clinical Global Impression–Severity score (1.83 vs 1.18; P < .001; effect size, 0.60) compared with placebo from baseline to 6 weeks.
Change in MADRS Score
Mixed model for repeated measures analysis (modified intent-to-treat population).
LS, least squares; MADRS, Montgomery-Asberg Depression Rating Scale.
*P < .05; **P < .01; ***P < .0001.
Reproduced with permission from T Suppes, MD, PhD.
At 6 weeks, the responder rate for the lurasidone group was also higher (64.8% vs 30.0%; P < .001; number needed to treat, 3).
The mean change from baseline to 6 weeks in the Hamilton Anxiety Rating Scale was −5.4 with placebo and −9.9 with lurasidone (P < .0001) and in the Young Mania Rating Scale, −5.4 and −7.0, respectively (P < .0001). The Sheehan Disability Scale was significantly reduced with lurasidone vs placebo (−11.2 vs −6.4; P < .001), and 3 of its subscales (work, social life, family disability) were also significantly reduced (P < .001).
Adverse events resulting in discontinuation were reported in 2.8% of patients receiving lurasidone, compared with 4.9% of those receiving placebo. Nausea was the only adverse event reported, with an incidence ≥ 2% (and greater than placebo) in patients receiving lurasidone (6.4% vs 2.0%). The incidence of somnolence, including hypersomnia, sedation, and somnolence, was 5.5% and 1.0% in the lurasidone and placebo groups, respectively.
This is the first reported placebo-controlled trial in adults with MDD with mixed features, Dr Suppes said, and the results demonstrate lurasidone’s efficacy in reducing depressive symptoms in these patients. The safety and tolerability of lurasidone in this study were also consistent with those previously reported in clinical trials in bipolar depression and schizophrenia.
- © 2015 SAGE Publications