Summary
The Kids B-LONG study was conducted to evaluate the pharmacokinetics, safety, and efficacy of recombinant human coagulation factor IX Fc fusion protein (rFIXFc) in pediatric subjects with hemophilia B. rFIXFc is considered safe and efficacious for prophylaxis and the control of acute bleeding in children age < 12 years.
- pediatric
- hemophilia B
- factor IX
- recombinant human coagulation factor IX Fc fusion protein
- hemophilia treatment
- hematology clinical trials
The Kids B-LONG study [NCT01440946] was conducted to evaluate the pharmacokinetics, safety, and efficacy of recombinant human coagulation factor IX Fc fusion protein (rFIXFc) in previously treated pediatric subjects with hemophilia B. Kathelijn Fischer, MD, PhD, University Medical Center Utrecht, Utrecht, The Netherlands, provided a summary of the Kids B-LONG study results.
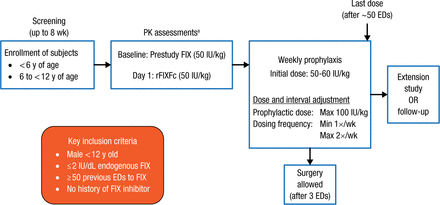
Eligible subjects were boys aged < 12 years with ≤ 2 IU/dL endogenous factor IX (FIX) and with ≥ 50 previous exposure days (EDs) to FIX without a history of FIX inhibitor. The study schematic is illustrated in Figure 1. All subjects had pharmacokinetic assessment with FIX 50 IU/kg and then with rFIXc 50 IU/kg before this study. Then, all received weekly prophylaxis with rFIXFc and were followed for 50 EDs. Surgery was allowed after 3 EDs.
Kids B-LONG Study Design
ED, exposure day; FIX, factor IX; PK, pharmacokinetic; rFIXFc, recombinant factor IX Fc fusion protein.
aA 96-hour washout period with no FIX treatment was required prior to administration of prestudy FIX, 28 ± 7 d prior to rFIXFc dosing at baseline, and prior to administration of rFIXFc on day 1; in younger children and subjects who required a second washout attempt, a 72-hour washout period was permitted.
Reproduced with permission from K Fischer, MD, PhD.
Thirty subjects were enrolled in the study; 15 patients were < 6 years old, and 15 were between 6 and 12 years. All subjects were treated with FIX prophylaxis before entering the study, and 77% were receiving FIX ≥ 2 times per week. Twenty-seven subjects (90%) completed the study, with a median study duration of 49.4 weeks. A total of 24 subjects (80%) had at least 50 EDs of rFIXFc.
Pharmacokinetic analyses indicated that rFIXFc had an increased half-life and reduced clearance in children compared with FIX products administered in the earlier phase of pharmacokinetic assessment. Also, incremental recovery with rFIXFc was similar to or slightly better than that with FIX.
No inhibitors or anti-rFIXFc antibodies developed in study subjects. The majority of subjects (86.7%) had ≥ 1 adverse event, the most common being nasopharyngitis (23.3%) and fall (20.0%). One mild nonserious adverse event of decreased appetite was considered to be related to study treatment; all other adverse events were considered unrelated to rFIXFc. Eleven serious adverse events occurred in 4 subjects during the study; none was considered related to the study drug. There were no discontinuations from the study because of adverse events.
No changes were made to the dosing interval in 97% of subjects. The median prophylactic dose was 59.4 IU/kg/wk (interquartile range [IQR], 53.0 to 64.8) in subjects aged < 6 years and 57.8 IU/kg/wk (IQR, 51.7 to 65.0) in subjects aged 6 to < 12 years.
Thirty-three percent of subjects had no bleeding episodes, and there were no joint bleeds in 63% of subjects. The overall median annualized bleeding rate was 2.0 (IQR, 0.0 to 3.1). In acute bleeding episodes, 75.0% were controlled with 1 infusion, and 91.7% were controlled with 1 or 2 infusions (median dose per infusion, 63.5 IU/kg [IQR, 48.9 to 99.4]). After the first infusions, 88.7% had an excellent or good response. Although no major surgeries were performed with rFIXFc, 3 minor surgeries were performed in 2 subjects. Excellent hemostatic response was achieved in all surgical cases. Physical activity remained the same or increased in 77% of study subjects. In her concluding remarks, Dr Fischer said she believes these data support the potential for extended-interval dosing with low bleeding rates in this population.
- © 2015 SAGE Publications