Summary
Agents that reverse the effect of vitamin K antagonists include plasma, vitamin K, and prothrombin complex concentrate. Hemostasis is most likely to be achieved by using a prothrombin complex concentrate. Guidelines for reversing the effect of direct-acting anticoagulants are based on guidelines that reflect the paucity of high-quality evidence.
- warfarin
- direct-acting anticoagulants
- vitamin K antagonists
- vitamin K
- plasma
- prothrombin complex concentrate
- rivaroxaban
- edoxaban
- dabigatran
- apixaban
- reversal
- cardiology & cardiovascular medicine clinical trials
- cardiology & cardiovascular medicine guidelines
Despite the availability of the direct-acting oral anticoagulants, millions of patients still take vitamin K antagonists (VKAs) for the treatment and prevention of thromboembolic events (TEEs).
Ravi Sarode, MD, University of Texas Southwestern Medical Center, Dallas, Texas, USA, began the session by highlighting guidelines from The American College of Chest Physicians regarding the management of VKA-associated bleeding [Holbrook A et al. Chest. 2012] that include the following:
INR between 4.5 and 10, with no evidence of bleeding: Do not routinely use vitamin K.
INR > 10: Administer vitamin K.
VKA-associated bleeding: Initiate rapid reversal of anticoagulation with 4-factor prothrombin complex concentrate (PCC).
Three agents are currently used to reverse the effect of VKAs: vitamin K, plasma, and PCCs. Oral vitamin K has a slow onset of action and is not feasible for patients who are actively bleeding. Because intravenous (IV) vitamin K is absorbed more predictably than the subcutaneous formulation, the IV option is preferable. While 5 to 10 mg of vitamin K is recommended for reversal, a lower dose (1 to 2 mg) may be a safer option in some patients to avoid VKA resistance.
There are limitations to using plasma to urgently reverse the effects of VKAs. Patients must be matched by blood group, and frozen plasma must be thawed. The recommended dose is 10 to 30 cc/kg, which can cause volume overload in some patients. Additionally, plasma takes a long time to transfuse, and there is a risk of transfusion-related adverse events.
Dr Sarode next reviewed the nonactivated PCCs. These include 4-factor PCCs, which contain all vitamin K–dependent factors, and 3-factor PCCs, which are devoid of factor VII and contain almost no protein C and protein S. Prothrombin complex concentrates can be administered promptly because of their relatively small infusion volume, and they require no thawing time or blood group matching [Sarode R et al. Circulation. 2013].
Dr Sarode highlighted a Phase IIIB noninferiority trial comparing a 4-factor PCC with plasma in adults taking a VKA who presented with major bleeding [Sarode R et al. Circulation. 2013]. The primary end points were 1) 24-hour hemostatic efficiency from the start of the infusion, and 2) INR correction (≤ 1.3) half an hour after infusion. Dosing was based on baseline INR and body weight. Patients were excluded if they had acute trauma; a history of TEEs, myocardial infarction, disseminated intravascular coagulation, or stroke; suspected or confirmed sepsis; or a history of antiphospholipid antibody syndrome.
Effective hemostasis was achieved in a greater percentage of patients taking 4-factor PCC (72.4%) than plasma (65.4%) and demonstrated noninferiority (difference, 7.1% [95% CI, −5.8 to 19.9]). Four-factor PCC was superior to plasma in reducing INR (62.2% vs 9.6%, respectively; difference, 52.6% [95% CI, 39.4 to 65.9]). Although the study was not powered to demonstrate significant differences in safety outcomes between the 2 groups, no unexpected safety signals were seen.
There are concerns regarding the association of PCCs with TEEs. Because patients with TEEs were excluded from their trial, Dr Sarode and his group completed a real-world retrospective analysis of 113 patients who had undergone warfarin reversal with 4-factor PCC [Joseph R et al. ASH 2014 (abstr 1561)]. Although the incidence of TEEs was similar to that found in the published studies, 2 patients died from TEEs possibly related to administration of PCC.
Dr Sarode concluded by emphasizing that VKAs will continue to be an important therapeutic option for patients requiring anticoagulation therapy; therefore, there is a need for randomized clinical trials that will identify and confirm the ideal hemostatic PCC dose.
Sunny Dzik, MD, Massachusetts General Hospital, Boston, Massachusetts, USA, then provided a literature update on whether PCCs can reverse oral factor Xa inhibitors. He began by noting the plethora of guidelines for urgent reversal of oral Xa inhibitors that often make reference to the use of PCCs as potential reversal agents.
Many of the guideline statements that recommend use of PCCs reference 1 small, randomized controlled trial published in 2011 [Eerenberg ES et al. Circulation. 2011]. In this trial of 12 healthy males, volunteers received rivaroxaban 20 mg BID for 2 days followed by a bolus of either saline or Cofact, a PCC formulation not available in North America. Rivaroxaban resulted in prolongation of the prothrombin time (PT), which was immediately and completely reversed following infusion of the PCC.
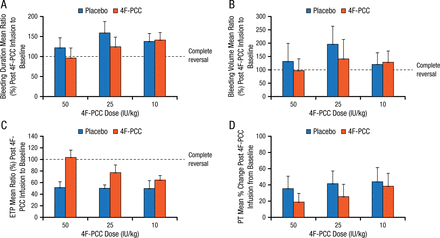
Dr Dzik then highlighted another small study of similar design, which showed that administration of the only 4-factor PCC available in the United States to volunteers taking rivaroxaban failed to completely reverse the PT and had no greater effect on anti-Xa levels than did saline [Levi M et al. J Thromb Haemost. 2014]. In another small phase I study in volunteers who took 1 dose of endoxaban, local bleeding following deliberate punch biopsy was not significantly different in those receiving PCC compared with placebo (Figure 1) [Zahir H et al. Circulation. 2015].
Effect of 4F-PCC vs Placebo on Bleeding Time
Effect of treatment with 4F-PCC or placebo on the mean ratio of post–4F-PCC infusion to baseline from bleeding duration (minutes) (A), baseline bleeding volume (mL) (B), ETP (nM×min) (C), and for mean percent change in PT (seconds) (D). Error bars represent upper limit of the 95% CI.
CI indicates confidence interval; ETP, endogenous thrombin potential; 4F-PCC, 4-factor prothrombin complex concentrate; and PT, prothrombin time.
Reprinted from Zahir H et al., Edoxaban effects on bleeding following punch biopsy and reversal by a 4-factor prothrombin complex concentrate, Circulation. 2015, Volume 131, Issue 1, Pages 82–90, with permission from American Heart Association, Inc.
Dr Dzik then briefly presented new information on andexanet alfa, which is under development as a reversal agent for direct fXa inhibitors. He presented data showing the effect on anti-Xa levels observed in a phase 2 study that examined whether andexanet alfa could reverse the anticoagulation effect of edoxaban [Crowther M et al. ASH 2014 (abstr 4269)]. Immediately after a 600- or 800-mg bolus of andexanet alfa, anti-Xa activity decreased dose-dependently by 52% and 73%, respectively, from the pre-andexanet level and returned to placebo levels at approximately 2 hours. In addition, andexanet alfa reversed thrombin generation and the prolongation of clotting times. There were no thrombotic events or other serious adverse events noted.
As a takeaway message, Dr Dzik encouraged clinicians to consider the clinical evidence as they make decisions regarding the use of PCCs to reverse anticoagulation, and highlighted the need for controlled trials of reversal using clinically meaningful end points.
- © 2015 SAGE Publications