Summary
Thrombosis in the small veins is not trivial and requires treatment. Fondaparinux in superficial vein thrombosis of the legs decreases the risk of recurrence, deep vein thrombosis, and pulmonary embolism. Treatment of cerebral vein thrombosis with low molecular weight heparin initially, followed by oral anticoagulation, reduces the risk of death and dependency.
- thrombosis
- superficial vein thrombosis
- cerebral venous thrombosis
- anticoagulation
- thrombotic disorders
- cardiology & cardiovascular medicine clinical trials
- cardiology & cardiovascular medicine guidelines
Recommendations for treatment of superficial vein thrombosis (SVT) of the legs now include anticoagulation; however, the low quality of evidence to support this recommendation and the uncertainty of cost-effectiveness have resulted in the question: Do patients with SVT require treatment? Hervé Décousus, MD, CHU de Saint-Etienne, Saint Etienne, France, discussed the management of spontaneous acute SVT of the legs.
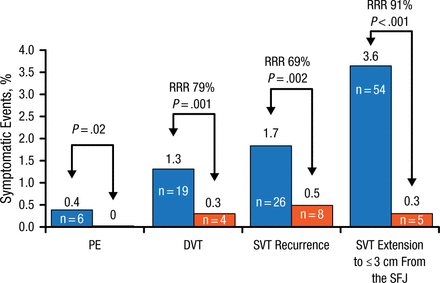
Several studies have demonstrated that deep vein thrombosis (DVT) and pulmonary embolism (PE) are present in 25% to 29% of patients who present with primary or secondary SVT [Frappé P et al. J Thromb Haemost. 2014; Galanaud JP et al. Thromb Haemost. 2011; Décousus H et al. Ann Intern Med. 2010]. In addition, patients with isolated SVT are at an increased risk of developing a subsequent DVT or PE between 3 and 6 months, with rates of up to 3.1% and 0.9% for DVT and PE, respectively [Décousus H et al. J Thromb Haemost. 2015]. Patients with SVT are at risk of SVT extension, with 3.6% of extensions occurring ≤ 3 cm from the saphenous-femoral junction and 3.7% occurring farther away [Leizorovicz A et al. Blood. 2013]. Importantly, SVT extension is associated with the development of a subsequent DVT or PE. Therefore, Prof Décousus recommends that patients who present with SVT should be evaluated for PE symptoms and an ultrasound should be conducted to determine if DVT is present.
Patients with SVT should receive anticoagulation therapy to prevent symptomatic thromboembolism. The randomized, double-blind STENOX trial [Superficial Thrombophlebitis Treated by Enoxaparin Study Group. Arch Intern Med. 2003] demonstrated that low molecular weight heparin (LMWH) for 12 days reduced the rate of DVT and SVT for over 80 days; however, most events occurred after LMWH was stopped. This indicates that anticoagulation is effective, but 12 days is not adequate in duration. Furthermore, the VESALIO [VESALIO Investigators Group. J Thromb Haemost. 2005] and STEFLUX trials [Cosmi B et al. J Thromb Haemost. 2012] demonstrated that 30 days of LMWH is also insufficient. By contrast, the CALISTO trial [Décousus H et al. N Engl J Med. 2010] demonstrated that 2.5 mg of fondaparinux for 45 days reduced the risk of death or symptomatic thromboembolism without increasing the risk of bleeding and did not cause catch-up phenomena to occur. In addition, fondaparinux for 45 days reduced the risk of PE, DVT, SVT recurrence, and SVT extension ≤ 3 cm from the saphenous-femoral junction (Figure 1). In the CALISTO trial, even patients with no known risk factors for thromboembolism derived benefit from fondaparinux therapy for 45 days.
Effect of 45 Days of Fondaparinux on Thromboembolism Risk After SVT
Some patients had more than one event. Death: fondaparinux, n = 2 (cancer); placebo, n = 1 (acute heart failure).
DVT, deep vein thrombosis; PE, pulmonary embolism; RRR, relative risk reduction; SFJ, saphenous-femoral junction; SVT, superficial vein thrombosis.
Source: Décousus H et al. N Engl J Med. 2010.
Reproduced with permission from H Décousus, MD.
Therefore, the current American College of Chest Physicians recommendations for the treatment of SVT of the legs suggest a prophylactic dose of fondaparinux or LMWH for 45 days, with 2.5 mg of fondaparinux being the preferred drug [Kearon C et al. Chest. 2012]. In addition, a Cochrane review found that 2.5 mg of fondaparinux for 45 days for the treatment of SVT was a valid treatment, but the authors could not reach a conclusion regarding LMWH, unfractionated heparin, or nonsteroidal anti-inflammatory drugs [Di Nisio M et al. Cochrane Database Syst Rev. 2013].
However, there are still unanswered questions regarding the optimal treatment of SVT. For example, the optimal dose and duration of LMWH has not yet been established, there are currently no data regarding the safety and efficacy of the new oral anticoagulants, and fondaparinux has not been tested in patients who are pregnant or in patients with cancer or a recent history of venous thromboembolism (VTE).
Another important small vein thrombosis is that of the cerebral veins and dural sinuses. Cerebral venous thrombosis (CVT) has a much lower incidence than DVT or PE, but it occurs more frequently in children and younger adults. Jonathan M. Coutinho, MD, PhD, Academic Medical Center, Amsterdam, The Netherlands, discussed the epidemiology, clinical manifestations, and management of CVT.
CVT encompasses sinus thrombosis and cerebral venous thrombosis, which frequently occur in the same patient. Sinus thrombosis causes intracranial hypertension due to decreased cerebrospinal fluid absorption (Table 1). By contrast, cerebral venous thrombosis causes site-specific congestion leading to edema, and potentially infarction and hemorrhage.
Common Signs and Symptoms of Cerebral Venous Thrombosis
The risk factors of CVT are partly similar to those of VTE, such as thrombophilia, cancer, and pregnancy [Lauw MN et al. Semin Thromb Haemost. 2013]. However, CVT-specific risk factors include neurosurgery, head trauma, lumbar puncture or epidural anesthesia, and local infections [Saposnik G et al. Stroke. 2011]. Diagnosis requires magnetic resonance imaging (MRI) and magnetic resonance venography (MRV), or computed tomography (CT) venography in centers without access to MRV or for patients who cannot undergo an MRI scan. MRI is superior to CT for detection of concomitant brain parenchymal lesions (Table 2).
Potential Concomitant Parenchymal Lesions Due to Cerebral Venous Thrombosis
Anticoagulation with LMWH is currently the standard treatment to reduce the risk of death or dependency. This is based on data from 2 small trials that, when taken together, resulted in a risk ratio of 0.46, albeit with a wide confidence interval (95% CI, 0.16 to 1.31) and lack of statistical significance (P = .12) [Coutinho J et al. Cochrane Database Syst Rev. 2011]. However, Dr Coutinho pointed out that in the heparin arm, none of the patients developed a new intracerebral hemorrhage despite many having hemorrhage at baseline, and 2 patients in the placebo arm developed probable PE. Endovascular treatment should be used only in severe cases; however, the ongoing TO-ACT trial should answer the question of when thrombolysis or thrombectomy should be used [Coutinho JM et al. Int J Stroke. 2013].
The standard treatment for ambulatory patients after CVT is a vitamin K antagonist. Although international guidelines recommend oral anticoagulation for 3 to 6 months in provoked cases and 6 to 12 months for unprovoked cases, the optimal duration is still not established [Saposnik G et al. Stroke. 2011; Einhäupl K et al. Eur J Neurol. 2010]. There are little data for use of nonwarfarin oral anticoagulants in CVT, although case series on this topic were recently published (PubMed ID 25070963).
With adequate treatment, the prognosis of patients with CVT is quite good, with only 15% to 20% having permanent disability and 5% to 10% experiencing death.
- © 2015 SAGE Publications