Summary
It becomes apparent that thrombotic thrombocytopenia purpura is an acute life-threatening condition that can be complicated with multiple recurrences and long-term microvascular damages. In contrast with previous thought, thrombotic thrombocytopenia purpura does not appear to pose an appreciable risk in pregnancy. The new modality of treatment options may help to reduce the severity of acute illness and prevent further relapse.
- thrombotic thrombocytopenia purpura
- rituximab
- plasma exchange
- microthrombus
- von Willebrand factor
- pregnancy complications
- other diseases of blood & blood-forming organs
Thrombotic thrombocytopenia purpura (TTP) is rare but serious. It is characterized by renal failure, thrombocytopenia, and features of microangiopathic hemolytic anemia, including the presence of red cell fragments. TTP can be congenital or acquired. Pregnancy, drugs, infection, and cancer can trigger the acquired form of TTP. Treatment of both congenital and acquired TTP has advanced and new options are becoming available that may lessen the need for plasma-based therapy and the risks of immune suppression, according to Spero R. Cataland, MD, Wexner Medical Center at The Ohio State University, Columbus, Ohio, USA.
Both congenital TTP and acquired TTP feature defective cleavage of von Willebrand factor (vWF) by dysfunctional ADAMTS13, a zinc-containing metalloproteinase with a thrombospondin type 1 motif. This results in the formation of microthrombi that impair oxygen delivery to a variety of organs [Sadler JE. Blood. 2008]. Deficient ADAMTS13 alone is insufficient to cause TTP. Moreover, recovery of ADAMTS13 activity does not always achieve clinical remission [Sadler JE. Blood. 2008]. Low ADAMTS13 activity during remission can increase the risk of relapse [Jin M et al. Brit J Haematol. 2008; Peyvandi F et al. Haematologica. 2008], but this is not always true [Cataland SR et al. Eur J Haematol. 2009].
Treatment includes daily plasma exchange. Despite recovery from the acute episodes, many patients may develop recurrence. In Dr Cataland’s experience, prophylactic immunosuppression is undertaken in patients with ≥ 2 measurements of ADAMTS13 activity < 10% and ≥ 2 prior episodes of TTP, with the assumption that these patients are at greatest risk for relapse. Immunosuppression does carry risks concerning susceptibility to infection.
Rituximab targets the CD20 receptor on B lymphocytes, thus reducing the production of antibodies against ADAMTS13 [Scully M et al. Blood. 2011]. Rituximab may prevent recurrence in acquired TTP [Hie M et al. Blood. 2014; Westwood JP et al. J Thromb Haemost. 2013]. Still, there are issues to be resolved, including cost of treatment, a lack of placebo controls in the published data, and a clearer definition of the patients at greatest risk for relapse so that only those who would be most likely to benefit would receive the treatment.
Splenectomy may also prevent TTP recurrence in patients who are refractory to plasma exchange [Dubois L, Gray DK. Can J Surg. 2010].
Treatments being evaluated include the use of ARC1779, a nucleic acid macromolecule that binds to a vWF domain. The binding curbs prothrombotic activity and the formation of microthrombi [Cataland SR et al. Am J Hematol. 2012]. While patient data are limited at this point, the hope is that this approach could shorten the time needed for plasma exchange and so lessen the reliance on the plasma-based approach. Another monoclonal antibody called caplacizumab targeting vWF, is administered intravenously as a loading dose, followed by daily subcutaneous doses during and after plasma exchange. Finally, recombinant ADAMTS13 has entered clinical study and has the potential for therapeutic value in both congenital and acquired TTP [Schiviz A et al. Blood. 2012; Plaimauer B et al. J Thromb Haemost. 2011].
Sara K. Vesely, PhD, University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma, USA, has been part of a team that established a population-based, inception cohort TTP registry that includes prospectively collected data on patients receiving plasma exchange because of TTP, hemolytic uremic syndrome, or thrombotic microangiopathy.
Data presented were among patients with TTP with ADAMTS13 activity < 10% at presentation. In the rest of the summary, these patients are described as TTP patients for simplicity.
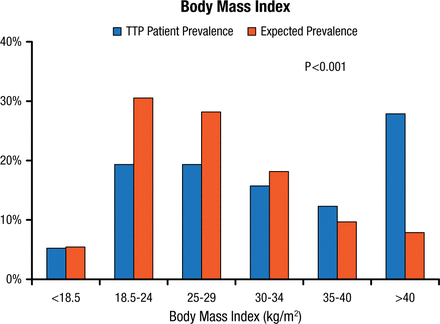
Comparison of registry data with data from the US National Health and Nutrition Examination Survey (NHANES) adjusted for age, sex, and race has revealed a significantly higher body mass index for TTP registry patients as compared with the US expected values (Figure 1) [Deford CC et al. Blood. 2013].
Prevalence of Categories of Body Mass Index Among TTP Patients and Expected Prevalence From NHANES Data Adjusted for Age, Sex, and Race
TTP, thrombotic thrombocytopenic purpura.
Reprinted from Deford CC et al. Multiple major morbidities and increased mortality during long-term follow-up after recovery from thrombotic thrombocytopenic purpura. Blood 2013;122(12)2023-2029. With permission from the American Society of Hematology.
The Oklahoma cohort highlights the heterogeneity in the clinical presentation of ADAMTS13 deficiency. Of 80 patients, 52% (n = 42) had severe neurological abnormalities but 30% (n = 24) had no neurological manifestations. Similarly, 40% (n = 32) presented with renal insufficiency but 51% (n = 41) had normal kidney function.
In the long term, relapse tends to occur within 3 years of remission. About one-half of the patients experienced > 1 relapse. With the advances in treatment including greater use of steroids and rituximab during the initial acute episode, the recurrence rate may have been reduced; of the 34 patients diagnosed after 2005, only 18% have relapsed.
While a low ADAMTS13 level during remission may precede a relapse, the risk of relapse in patients with ADAMSTS13 deficiency is low, according to data from the Oklahoma registry (3 of 18 patients relapsed within 1 year of an ADAMTS13 activity < 10%).
Pregnant women with a history of TTP are deemed to be at risk for recurrence and miscarriage [Ferrari B et al. Blood. 2014]. On the contrary, the Oklahoma cohort data revealed that TTP during pregnancy poses a low risk of fetal death. Women with TTP who desire to become pregnant need not be discouraged because of fetal health concerns. However, TTP is a risk factor for preeclampsia in a subsequent pregnancy (Table 1).
Risk of Preeclampsia in Subsequent Pregnancy in Women With TTP
TTP is not only a life-threatening acute condition, but it is also associated with long-term sequelae including impaired cognition, depression, systemic lupus erythematosus, hypertension, impaired kidney function, and early mortality [Deford CC et al. Blood. 2013; Kennedy AS et al. Transfusion. 2009]. The cognition deficits are characteristics of diffuse, subcortical, microvascular disease [Kennedy AS et al. Transfusion. 2009].
- © 2015 SAGE Publications