Summary
Multivariate analysis shows that > 2000 patients with hepatitis C virus who received 3 direct-acting antivirals and had <4 predictors of negative response experienced 12-week sustained virologic response rates > 95%. Analysis of those that received the label-recommended regimen found that the only 2 negative predictors associated with significantly lower response rates are obesity and genotype 1a disease. Even those with both factors achieved sustained virologic response rates of 95% at week 12.
- hepatitis C virus
- subgenotype
- negative predictors
- ombitasvir
- paritaprevir
- dasabuvir
- SAPPHIRE-I
- SAPPHIRE-II
- PEARL-II
- PEARL-III
- PEARL-IV
- TURQUOISE-II
- gastroenterology clinical trials
The multitargeted regimen of 3 direct-acting antivirals—ombitasvir (an NS5A inhibitor), dasabuvir (a nonnucleoside NS5B RNA polymerase inhibitor), and paritaprevir (an NS3/4A protease inhibitor boosted with ritonavir), with or without ribovarin—has demonstrated high efficacy in 6 phase 3 clinical trials encompassing > 2000 patients with genotype 1 hepatitis C virus (HCV; Table 1).
Overview of Treatment Regimens and SVR12 Rates in 6 Phase 3 Trials
Nancy Reau, MD, The University of Chicago Medicine, Chicago, Illinois, USA, presented a composite review of data from the 6 trials that examined whether multiple predictors of response had a meaningful impact on sustained virologic response at week 12 (SVR12). These predictors—traditionally associated with less robust clinical outcomes in patients with HCV—include cirrhosis, high baseline viral load, weight, IL28B non-CC genotype, and prior treatment failure.
A multivariate stepwise logistic regression was performed on data from the 2053 patients who participated in all 6 trials. The analysis included 22 continuous and categorical variables performed on 2 populations: (1) all patients who had data available regardless of whether they had received a label-recommended regimen and (2) patients who received a label-recommended regimen by HCV subgenotype and cirrhosis status.
Analysis of the entire cohort found that while genotype 1a disease was independently and significantly associated with reduced SVR12 (94%; P < .001), the SVR12 rate for genotype 1b was 99%. Other factors associated with significantly lower SVR12 included higher weight at baseline (P = .007), IL28B TT genotype (P = .034), Hispanic/Latino ethnicity (P = .013), and higher baseline HCV RNA (P = .041). Traditional factors associated with lower cure rates, such as the presence of cirrhosis and prior treatment response, were not associated with lower SVR rates.
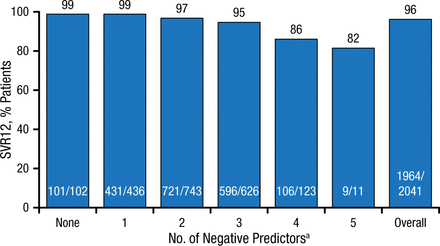
The SVR12 rates for the entire cohort by the cumulative number of individual negative predictors are shown in Figure 1.
SVR12 Rates per Cumulative Number of Negative Predictors
HCV, hepatitis C virus; SVR12, sustained virologic response for 12 wk.
aNegative predictors: HCV genotype 1a, weight ≥ 75 kg, IL28B TT, Hispanic/Latino, HCV RNA ≥ 800 000 IU/mL.
Reproduced with permission from N Reau, MD.
Among > 2000 patients in the studies, 1083 received the label-recommended regimen (Table 2). The overall rate of SVR12 among those patients was 97% (n = 1052), with rates similarly high regardless of whether cirrhosis was present. Only 1 patient with a genotype 1b infection did not achieve SVR12. Of the 31 patients who did not achieve SVR12, 19 (1.8%) had on-treatment breakthrough or posttreatment relapse; 12 (1.1%) discontinued treatment prematurely. Of these 12 discontinuations, 3 were due to adverse events. Of the 22 variables, only body mass index and HCV genotype 1a disease had a negative impact in the multivariate stepwise analysis.
Label-Recommended Regimens
According to Dr Reau, these data suggest that the regimen of 3 direct-acting antivirals with or without ribovarin confers high SVR12 rates and offers an effective treatment option for patients with HCV who present with factors previously thought to have an negative impact on treatment response.
- © 2015 SAGE Publications