Summary
Metabolic surgery (gastric restriction [sleeve gastrectomy and gastric banding], a combination approach using Roux-en-Y gastric banding, and the malabsorption method using a duodenal switch) can effectively and safely reduce weight, achieve diabetes remission, reduce comorbidities, and improve quality of life in obese individuals.
- metabolic surgery
- obesity
- gastric banding
- duodenal switch
- sleeve gastrectomy
- type 2 diabetes mellitus
- endoluminal devices
- endoscopic bariatric therapies
- endobarriers
- glycemic control
- gastroenterology procedures
The prevalence of obesity and diagnosed diabetes is a major problem in the United States. As of 2013, 26% of adults had a body mass index (BMI) > 30 kg/m2, and 9% (29.1 million) had diabetes mellitus (DM) [CDC’s Division of Diabetes Translation. http://www.cdc.gov/diabetes/data/. Accessed May 28, 2015]. Both of these problems have increased over the last 50 years. Matthew M. Hutter, MD, Massachusetts General Hospital, Boston, Massachusetts, USA, discussed the options to correct these problems and outcomes for metabolic surgery.
The potential for metabolic surgery to be effective for treatment of type 2 DM (T2DM) was first proposed by Pories in 1995. His suggestion was confirmed by a 2005 meta-analysis that showed that many patients with obesity undergoing metabolic surgery experienced resolution or improvement not only in DM but also in hyperlipidemia, hypertension, and obstructive sleep apnea. Dr Hutter noted that the importance of this surgery for metabolic diseases led to a change in terminology from bariatric surgery to metabolic surgery.
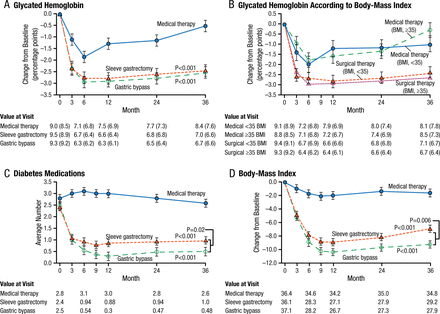
Options for metabolic surgery include bypass and the use of gastric bands and sleeve gastrectomy. In the United States, there is a trend toward decreasing the use of bypass and band procedures and increasing the use of the sleeve. The 3-year, randomized STAMPEDE trial [Schauer PR et al. N Engl J Med. 2014] reported that intensive medical therapy plus metabolic surgery (Roux-en-Y gastric bypass or sleeve gastrectomy) resulted in glycemic control in significantly more patients than did medical therapy alone (P < .001). Improved body weight, less use of glucose-lowering medications, and improved quality of life (QOL) were also more favorable for the metabolic surgery group (Figure 1).
Mean Changes in Parameters of Diabetes Control
Mean Changes in Measures of Diabetes Control from Baseline to 3 Years. Shown are the percentage change in glycated hemoglobin levels (Panel A), the percentage change in glycated hemoglobin levels according to body-mass index (BMI) (Panel B), the average number of diabetes medications during the study period (Panel C), and the changes in BMI (Panel D) over a 3-year period among patients receiving intensive medical therapy only, sleeve gastrectomy, or gastric bypass. I bars indicate standard errors. Mean values in each group are provided below the graphs; in Panels A and B, median values are also provided in parentheses. P values are for the comparison between each surgical group and the medical-therapy group in Panels A, C, and D. In Panel B, P=0.008 for the comparison between the surgical groups and the medical-therapy group for the subgroup of patients with a BMI of less than 35; P<0.001 for the comparison for the subgroup with a BMI of 35 or more.
From N Engl J Med, Schauer PR et al., Bariatric Surgery versus Intensive Medical Therapy for Diabetes—3-Year Outcomes, Volume No. 370, Page No. 2002-2013. Copyright © (2014) Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Although these are all effective procedures, studies have shown morbidity rates of 3% to 20% and mortality rates of 0.1% to 0.5% [ASGE/ASMBS Task Force on Endoscopic Bariatric Therapy. Gastrointest Endosc. 2011]. In a head-to-head comparison, favorable outcomes for bypass, sleeve, and band procedures differ depending on the study. In a meta-analysis of 11 studies to quantify the overall effects of metabolic surgery compared with nonsurgical treatment for obesity, metabolic surgery led to greater body weight loss, higher remission rates of T2DM and metabolic syndrome, greater improvements in QOL, and reductions in medication use [Gloy VL et al. BMJ. 2013].
One study showed that, among patients with DM, neither the choice of procedure nor baseline BMI impacted DM resolution [Panunzi S et al. Ann Surg. 2015]; however, a review reported that in patients with BMI < 35 kg/m2, metabolic surgery was associated with higher T2DM remission rates, a higher rate of glycemic control, and lower HbA1c levels compared with medical treatment [Müller-Stich BP et al. Ann Surg. 2015]. Follow-up ranged from 12 to 36 months.
These studies and others indicated that metabolic surgery is effective treatment not only for weight loss but also for achieving DM remission, improving metabolic syndromes, and achieving significant improvements in QOL.
Despite the known consequences of obesity and the availability of surgical treatment options, only a small percentage of patients eligible for surgery are being treated. Many of these patients are concerned with what they feel may be an invasive, painful procedure requiring a long recovery. Other options are available, however. Aurora D. Pryor, MD, Stony Brook Medicine, Stony Brook, New York, USA, discussed the newer endoluminal bariatric therapies.
Endoluminal bariatric treatment approaches are less painful and minimally invasive, reduce risk, and decrease recovery time and cost, but their risks and benefits need further study, as does the appropriateness of their disabilities, durabilities, and resource utilizations. The recommended weight loss thresholds are 5% of total body weight for treatment of early metabolic disease, 20% excess weight loss (EWL) for bridge therapy, and 25% EWL for primary therapy (less in the case of lower-risk procedures) [ASGE/ASMBS Task Force on Endoscopic Bariatric Therapy. Gastrointest Endosc. 2011].
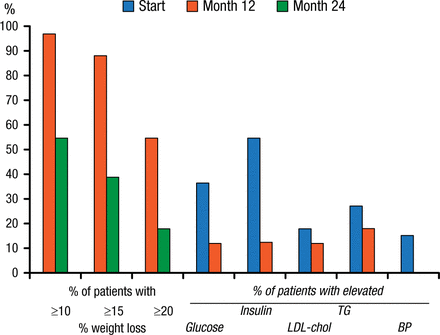
Space-occupying devices such as intragastric bubbles were first proposed in 1982. Their use was discontinued in 1989 after several trials showed no benefit over sham procedures as well as significant complications. The BioEnterics Intragastric Balloon device that is expected to receive FDA approval in 2015 is showing more promise. In a 2005 study that assessed this device, EWL at 6 months was 34%, with a 45% improvement in comorbidities. In another 2005 study, significant weight loss was noted at 1 year (Figure 2). At the end of the second balloon-free year, 47% of patients sustained a > 10% weight loss. Glucose, insulin, low-density lipoprotein cholesterol, and triglycerides were also reduced relative to baseline after 1 year of balloon therapy (Figure 2). The reshape balloon is also expected to garner approval in 2015.
Weight and Comorbidity Reductions Following Intragastric Balloon Therapy
Per protocol analysis. Percentage of patients achieving 10%, 15%, and 20% weight loss after 12 and 24 mo, and percentage of patients with elevated values for glucose (> 6 mmol/L), insulin (>20 IU/L), LDL-cholesterol (>4.5 mmol/L), triglycerides (TG) (>2 mmol/L), and elevated diastolic blood pressure (BP) (>100 mm Hg) at baseline (start) and after 1 year.
Reprinted from Gastrointest Endosc, Vol 61, Mathus-Vliegen EMH et al., Intragastric balloon for treatment-resistant obesity: safety, tolerance, and efficacy of 1-year balloon treatment followed by a 1-year balloon-free follow-up, Pages 19-27, Copyright (2005), with permission from American Society for Gastrointestinal Endoscopy.
Balloons may be endoscopically inserted or swallowed. Both require endoscopic retrieval. Another device is swallowed and dissolvable. A novel endoluminal device (TransPyloric Shuttle) is endoscopically positioned in the transpyloric area to delay gastric emptying, reduce caloric intake, and reduce weight [Marinos G et al. Surg Obes Relat Dis. 2014]. After 3 months, patients receiving the device had a 25% and 41% EWL at 3 and 6 months, respectively. At 6 months, total weight loss was about 15%.
Tansoral endoscopically placed tissue anchors to reduce stoma diameter and pouch volume, as well as the need for revisional gastric bypass surgery, have been shown to be feasible [Herron DM et al. Surg Endosc. 2008]. Suture anchors can be applied either by application or by apposition. In one study, a novel endoscopic duodenal-jejunal bypass liner after 24 weeks reduced weight and significantly improved HbA1c levels (P < .01) [de Jonge C et al. Obes Surg. 2013]. A European study has reported that the endoscopically placed duodenal-jejunal bypass liner (EndoBarrier Gastrointestinal Liner) is a feasible and safe noninvasive device with excellent short-term weight loss results, and has significant positive effects on T2DM [Schouten R et al. Ann Surg. 2010].
However, endobarriers have been associated with bleeding, pain, vomiting, and obstruction. Weight gain may also occur once the endobarrier is removed.
One of the newest endoscopic procedures is duodenal mucosal resurfacing by thermal mucosal ablation. It is unique in that it does not involve an indwelling device. Initial results of one study showed a 2% drop in HbA1c levels at 3 months, which were maintained for 6 months [Rodriguez L et al. IFSO 2014 (abstr OS22.01)].
Dr Pryor concluded that there are many devices and procedures in development that may help promote the resolution of metabolic disease independent of weight loss.
- © 2015 SAGE Publications