Summary
Eluxadoline, a µ-opioid receptor agonist and d-opioid receptor antagonist, exhibits rapid onset of action along with sustained efficacy over the 6-month treatment period as studied in the phase 3, double-blind, placebo-controlled IBS3001 and IBS3002 trials in patients with diarrhea-predominant irritable bowel syndrome.
- eluxadoline
- irritable bowel syndrome with diarrhea
- IBD3001
- IBS3002
- µ-opioid receptor agonist
- d-opioid receptor antagonist
- irritable bowel syndrome
- gastroenterology clinical trials
Irritable bowel syndrome with diarrhea (IBS-D), a common gastrointestinal (GI) disorder, is characterized by bloating, urgency, recurring abdominal pain, and loose, frequent stools in the absence of structural or biochemical abnormalities. Endogenous µ- and d-opioid receptors are critical for the normal function and sensation of the GI tract. A novel mixed µ-opioid receptor agonist and d-opioid receptor antagonist, eluxadoline, acts locally in the GI tract with low systemic absorption and oral bioavailability [Fujita W et al. Biochem Pharmacol. 2014].
William D. Chey, MD, University of Michigan Health System, Ann Arbor, Michigan, USA, discussed the results of IBS3001 [NCT01553591] and IBS3002 [NCT01553747], 2 randomized, double-blind, placebo-controlled, phase 3 trials that evaluated the sustained efficacy of twice-daily treatment with eluxadoline in adults with IBS-D.
Both studies randomly assigned adults 1:1:1 to placebo twice daily, eluxadoline 75 mg BID, or eluxadoline 100 mg BID. Both studies were 26 weeks, and efficacy was assessed at week 12 for the FDA and at week 26 for the European Medicines Agency. IBS3002, conducted in the United States, United Kingdom, Canada, and Puerto Rico, concluded at 26 weeks. For IBS3001, conducted in the United States, United Kingdom, and Canada, treatment was extended for an additional 26 weeks to assess the safety of long-term use.
Inclusion criteria comprised IBS-D (Rome III criteria), average worst abdominal pain (WAP) score > 3 (scale 0 to 10), average stool consistency Bristol Stool Scale (BSS) score ≥ 5.5 (scale 1 to 7), at least 5 days with a BSS score ≥ 5.5 during the 4-week screening period, and IBS-D global symptom score ≥ 2.0 (scale 0 to 4). Patients with inflammatory bowel or celiac disease, prior pancreatitis, sphincter of Oddi dysfunction, and other GI conditions or elevated lipase, alanine, or aspartate aminotransferases were excluded.
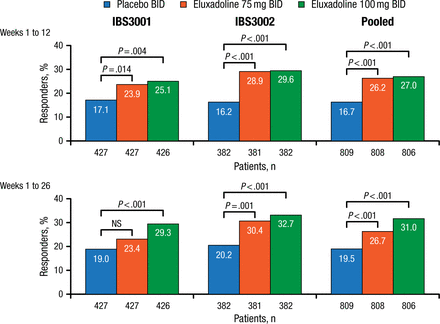
The composite efficacy end point was response, evaluated at weeks 1 through 12 (FDA primary end point) and weeks 1 through 26 (European Medicines Agency primary end point). Responders were defined as having an improvement in daily pain (WAP scores improved by ≥ 30% in the past 24 hours compared with average baseline pain) and daily stool consistency (BSS score < 5) on at least 50% of days.
Baseline characteristics of enrolled patients in IBS3001 (n = 1282) and IBS3002 (n = 1146) were comparable. Mean BSS and WAP were both > 6, indicating a severely affected population. Efficacy results are shown in Figure 1.
Primary Composite Efficacy End Point
NS, not significant.
Reproduced with permission from WD Chey, MD.
Efficacy of eluxadoline was seen within week 1, with maximum benefit achieved in weeks 4 to 6 and sustained over the 6-month efficacy evaluation period. Efficacy was seen in both men and women. Significantly more patients taking either eluxadoline dose reported ≥ 75% urgency-free days (P < .001) and decrease in bowel movement frequency (P < .001) than those taking placebo.
Adverse events were reported slightly more often by those in the eluxadoline groups; GI-related events were the most common.
Sphincter of Oddi spasm events occurred in 10 patients taking eluxadoline who had prior cholecystectomy; symptoms were reversible with withdrawal of drug. Pancreatitis occurred in 4 patients in the eluxadoline groups. No deaths or surgery resulted from these events.
To conclude, eluxadoline offers an effective new therapeutic option for patients with IBS-D; however, a risk of mild pancreatitis was seen.
- © 2015 SAGE Publications