Summary
A phase 2b randomized, double-blind, placebo-controlled study shows that naldemedine, a peripherally acting µ-opioid receptor antagonist, is effective in easing opioid-induced constipation compared to placebo. Phase 3 development is expected for the 0.2 mg/day naldemedine dose.
- naldemedine
- constipation
- opioids
- noncancer pain
- spontaneous bowel movements
- gastroenterology clinical trials
As described by Lynn Webster, MD, PRA Health Sciences, Salt Lake City, Utah, USA, a phase 2b, randomized, double-blind, placebo-controlled study revealed the benefit of naldemedine in easing constipation due to opioids given for relief of chronic noncancer pain.
The use of opioids for relief of noncancer pain has increased substantially over the last 20 years [Chou R et al. J Pain. 2009], but opioids can often result in constipation. Laxatives are a common recourse, but evidence for their effectiveness is scant and, in many patients, they do not provide satisfactory relief [Camilleri M et al. Neurogastroenterol Motil. 2014].
This trial evaluated naldemedine, a peripherally acting µ-opioid receptor antagonist that has been developed specifically for the relief of opioid-induced constipation. Patients meeting the enrollment criteria (n = 244) were randomized 1:1:1:1 (n = 61 for each group) to placebo or naldemedine 0.1, 0.2, or 0.4 mg/day. The primary efficacy end point was the mean change in the frequency of spontaneous bowel movements (SBMs) from the last 2 weeks of screening to the last 2 weeks of the up-to 28-day treatment period. Safety was also assessed.
Reported results represented patients in the modified intention-to-treat population who were assessed at least once (naldemedine 0.1 mg/d [n = 61], 0.2 mg/d [n = 59], and 0.4 mg/d [n = 57]). Patients in each of the 4 study arms were comparable at baseline in terms of age, sex, body mass index, weekly frequency of SBMs, and daily opioid dose. Naldemedine was rapidly absorbed and displayed a half-life that was compatible with once-daily use.
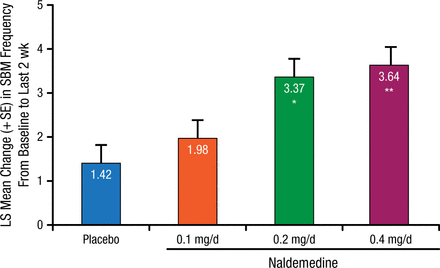
In the primary efficacy end point, naldemedine 0.2 and 0.4 mg/d produced significant differences in SBMs (3.37 and 3.64) compared with placebo (1.42; P = .0014 and P = .0003, respectively). Improvement was also evident for naldemedine 0.1 mg/d (1.98), but it was not significantly different from the placebo arm (Figure 1).
Primary Efficacy End Point
Modified intention-to-treat population: all randomized patients who received study drug had ≥ 1 postdose primary efficacy assessment completed.
LS, least squares; SBM, spontaneous bowel movement.
*P = .0014 vs placebo; **P = .0003 vs placebo and P = .6657 vs 0.2 mg (analysis of covariance with treatment group as a term and baseline value as a covariate).
Reproduced with permission from L Webster, MD.
A similar pattern was apparent for the secondary end point of SBM response rate (39.3%, 52.5%, 71.2%, and 66.7% for placebo, naldemedine 0.1, 0.2, and 0.4 mg/day, respectively; P = .0005 and P = .003 for naldemedine 0.2 and 0.4 mg/d, respectively). SBM frequency was significantly increased by naldemedine 0.2 and 0.4 mg/d by the first week of treatment and maintained through week 4 (P < .005 for both doses). Analyses of other secondary end points, such as relief of abdominal bloating and abdominal discomfort, as well as patient satisfaction, favored naldemedine, especially at 0.2 and 0.4 mg/d.
Treatment-emergent adverse events that developed occurred with similar frequency in the 4 trial arms. The incidence of gastrointestinal adverse events was greater in patients who were randomized to naldemedine (13.1%, 21.3%, 25.0%, and 34.4% in placebo and naldemedine 0.1, 0.2, and 0.4 mg/d, respectively).
Naldemedine treatment did not compromise the effectiveness of opioid pain relief, with no changes evident in pain scores or evidence of opioid withdrawal. The patterns over the 4-week trial were very similar in all 4 trial arms.
- © 2015 SAGE Publications