Summary
The Einthoven Lecture, presented by Prof Günter Breithardt, focused on atrial fibrillation (AF) and reviewed the history, mechanisms, and pathogenesis of AF. He focused on the association of metabolic syndrome and obesity with AF. These associations provide evidence that supports treatment strategies designed to prevent the development of AF.
- ablation
- arrhythmias
- atrial fibrillation
- epicardial adipose tissue
- epidemiology
- metabolic syndrome
- obesity
- primary prevention
- sleep apnea
- stroke
Since the first electrocardiogram recording of atrial fibrillation (AF) in the late 1800s and early studies showing the link between AF and stroke, much has been learned about this condition. Günter Breithardt, MD, Universitätsklinikum Münster, Münster, Germany, discussed how we are expanding our understanding of the influence of comorbid conditions on AF and how to use this understanding to improve patient care.
AF is important given its increasing prevalence in the current era. Although it is a global disease, the epidemiology of the condition varies by region. In 2010, the estimated global burden of AF was 33.5 million, with the United States and Canada having the highest prevalence, whereas Europe and Australia had the highest proportion of deaths associated with AF [Chugh SS et al. Circulation. 2014].
The clinical management of AF is evolving toward a more personalized approach based on validated parameters that encompass atrial morphology and damage, brain imaging, information on genetic predisposition, systemic or local inflammation, and markers for cardiac strain [Kirchhof P et al. Europace. 2013].
Of particular interest in the management of AF is the potential impact of appropriately treating comorbid conditions. Results from a 2011 study of 14 598 individuals (mean age, 54.2 years) who had been followed for 17 years in the ARIC study showed that 57% of incident AF events could be associated with risk factors such as hypertension, obesity, diabetes mellitus, smoking, and prior cardiac disease [Huxley RR et al. Circulation. 2011]. Thus, the majority of AF burden is potentially avoidable through the optimization of cardiovascular risk factor levels. As the number of comorbidities/risk factors for AF increases, there is higher likelihood that AF will progress to being permanent and the risk of stroke increases [Nabauer M et al. Europace. 2009].
Although the current European Society of Cardiology (ESC) guidelines encourage early treatment of AF using a variety of treatment modalities [Camm AJ et al. Eur Heart J. 2011], Prof Breithardt believes that we are still not paying enough attention to primary prevention. He is currently involved in the EAST trial, run by AFNET jointly with EHRA, a prospective study designed to test the hypothesis that an early, structured rhythm control therapy based on antiarrhythmic drugs and catheter ablation can prevent AF-related complications in patients with AF when compared with usual care.
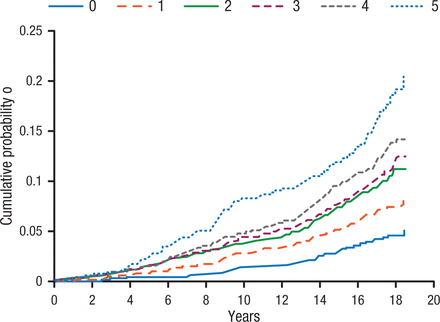
When studying AF, it is important not to bundle AF patients together as there are a variety of clinical subgroups, including a large group of patients with unclassified AF. Among these are patients with obesity, increased body mass index, and the metabolic syndrome. In a meta-analysis of nearly 6000 cases of AF, increasing body mass index was associated with a significant increase in AF incidence, whereas sustained weight loss was associated with significant reduction of AF burden [Pathak RK et al. J Am Coll Cardiol. 2015]. Importantly, as the number of components of the metabolic syndrome increase, so does the probability of AF (Figure 1) [Chamberlain AM et al. Am Heart J. 2010].
Incidence of Atrial Fibrillation Associated With Increasing Components of Metabolic Syndrome
Figure adjusted for the following covariates at baseline: age (45 to < 50, 50 to < 55, 55 to < 60, ≥ 60), sex, race, center, educational attainment, smoking status and cigarette-years of smoking (quartiles).
Reprinted from Chamberlain AM et al, Metabolic syndrome and incidence of atrial fibrillation among blacks and whites in the Atherosclerosis Risk in Communities (ARIC) Study, Am Heart J, 2010, Vol 159, Issue 5, Pages 850-856, with permission from 2010 Mosby, Inc.
Excess weight is also a predictor of sleep-disordered breathing, or sleep apnea, which is itself an independent predictor of stroke [Yaggi HK et al. N Engl J Med. 2005]. Indices of sleep-disordered breathing are positively correlated with visceral fat [Vgontzas AN et al. J Clin Endocrinol Metab. 2000], as well as higher plasma concentrations of the tumor necrosis factor-α and the proinflammatory cytokine interleukin-6, both of which are markers of inflammation [Vgontzas AN et al. Sleep Med Rev. 2005].
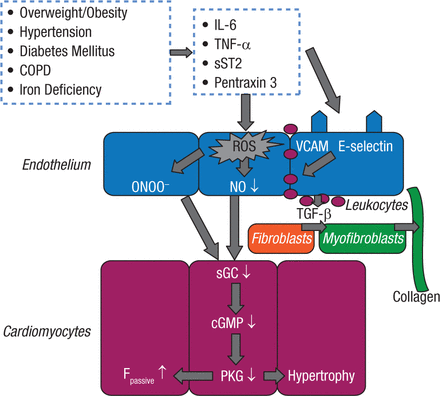
There is also a relationship between sleep-disordered breathing, overweight/obesity, and heart failure (HF). It has recently been suggested that HF with preserved ejection fraction (HFpEF) may be another manifestation of the metabolic syndrome in which myocardial dysfunction and remodeling are driven by inflammation (Figure 2) [Paulus WJ, Tschope C. J Am Coll Cardiol. 2013].
New Paradigm for Heart Failure With HFpEF: Importance of Comorbidities
Comorbidities induce a systemic proinflammatory state with elevated plasma levels of interleukin (IL)-6, tumor necrosis factor (TNF)-a, soluble ST2 (sST2), and pentraxin 3. Coronary microvascular endothelial cells reactively produce reactive oxygen species (ROS), vascular cell adhesion molecule (VCAM), and E-selectin. Production of ROS leads to formation of peroxynitrite (ONOO) and reduced nitric oxide (NO) bioavailability, both of which lower soluble guanylate cyclase (sGC) activity in adjacent cardiomyocytes. Lower sGC activity decreases cyclic guanosine monophosphate concentration and protein kinase G (PKG) activity. Low PKG activity increases resting tension (Fpassive) of cardiomyocytes because of hypophosphorylation of titin and removes the brake on prohypertrophic stimuli inducing cardiomyocyte hypertrophy. VCAM and E-selectin expression in endothelial cells favors migration into the subendothelium of monocytes. These monocytes release transforming growth factor b (TGF-b). The latter stimulates conversion of fibroblasts to myofibroblasts, which deposit collagen in the interstitial space. COPD = chronic obstructive pulmonary disease; HPEF = heart failure with preserved ejection fraction.
Reprinted from J Am Coll Cardiol. Vol 62, A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. Pages 263-7, Copyright 2013, with permission from American College of Cardiology Foundation.
Animal studies have also demonstrated the relationship between sleep apnea and AF. A recent study in rats showed that chronic obstructive sleep apnea (OSA) leads to AF-promoting cardiac remodeling, with conduction abnormalities related to connexin dysregulation and increased fibrosis playing a prominent role [Iwasaki YK et al. J Am Coll Cardiol. 2014].
Prof Breithardt noted that adipose tissue induces fibrosis in the atria, whereas visceral adipose tissue (pericardial fat) volume is highly associated with paroxysmal and persistent AF independent of traditional risk factors, including left atrial enlargement [Al Chekakie MO et al. J Am Coll Cardiol. 2010]. Pericardial fat is also associated with AF severity, chronicity, and LA volume. Additionally, it is also predictive of long-term AF recurrence after ablation [Wong CX et al. J Am Coll Cardiol. 2011]. Very recent experimental studies have shown that human epicardial adipose tissue, but not subcutaneous fat tissue, induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines, thereby creating a substrate for AF [Venteclef N et al. Eur Heart J. 2015]. Thus, epicardial adipose tissue may be an important modifier linking obesity, sleep apnea, and HF to AF. Higher plasma concentration of resistin, a hormone associated with obesity, inflammation, and HF, has been implicated in incident AF [Rienstra M et al. Am Heart J. 2012] and may be a good marker.
Knowledge of these cause-and-effect mechanisms supports new preventive measures. Sleep apnea as an expression of obesity can also predict the risk of recurrent AF after ablation and cardioversion, whereas the use of continuous positive airway pressure can reduce the incidence of OSA-related arrhythmias and AF [Abe H et al. Heart Vessels. 2010]. Other studies suggest that interventions can prevent or reduce AF occurrence. In overweight or obese patients undergoing weight and cardiometabolic risk factor management, significant reduction in AF symptom burden, severity scores, inflammation, and improved cardiac remodeling were reported [Abed HS et a. JAMA. 2013], as well as improved long-term success of AF ablation [Pathak RK et al. J Am Coll Cardiol. 2014].
In summary, obesity and its associated comorbidities are likely contributors to the expanding epidemic of AF. Patients with untreated OSA have a higher recurrence of AF after cardioversion, which can be reduced with continuous positive airway pressure. Weight reduction and cardiometabolic risk factor management also are associated with reductions in symptomatic AF burden, higher percentage of time in sinus rhythm after ablation, improvement in cardiac structure, and reductions in pericardial adipose tissue.
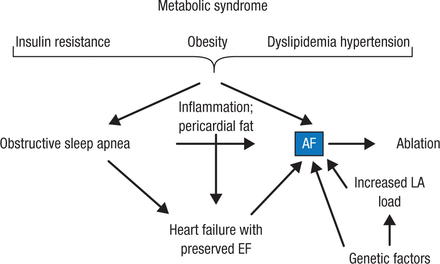
Figure 3 outlines Prof Breithardt’s current understanding regarding metabolic syndrome and AF.
Metabolic Syndrome and Atrial Fibrillation
AF, atrial fibrillation; EF, ejection fraction; LA, left atrium.
Reproduced with permission from G Breithardt, MD.
To translate this new knowledge into clinical practice, there is a need to highlight the impact of obesity on various cardiovascular entities such as myocardial infarction and vascular stroke, HFpEF, and sleep apnea, all of which lead to AF. In addition, it is important to align with the WHO and WHF targets of achieving a 25% relative reduction in premature mortality from noncommunicable diseases by 2025.
- © 2015 SAGE Publications