Summary
Three speakers presented information regarding the importance of differentiating atrial high-rate episodes from atrial fibrillation, the importance of developing patient registries of atrial fibrillation, and recommendations for using oral anticoagulants in patients who undergo atrial ablation or closure of the left atrial appendage.
- atrial fibrillation
- atrial high-rate episodes
- oral anticoagulants
- left atrial appendage
- atrial ablation
- patient registries
- stroke
- warfarin
- cardiology & cardiovascular medicine clinical trials
- interventional techniques & devices
In a joint session of the European Heart Rhythm Association and the Sociedade Brasileira de Arritmias Cardiacas, 3 presenters offered perspectives on topics ranging from atrial high-risk episodes (AHREs) and stroke, the value of patient registries, and anticoagulation strategies following atrial ablation or closure of the left atrial appendage (LAA).
According to Paulus Kirchhof, MD, University of Birmingham, Birmingham, United Kingdom, silent, undiagnosed atrial fibrillation (AF) is a common cause of stroke. He reviewed the MonDAFIS trial [NCT02204267], an ongoing study investigating the burden of paroxysmal AF in acute stroke patients with no known AF. Patients are randomized to either an experimental arm (prolonged electrocardiogram monitoring) or a regular treatment arm (long-term electrocardiogram monitoring for a maximum duration of 7 days). The primary outcome measures are the number of patients on oral anticoagulants (OACs) at 12 months and the number of patients on OACs at 12 months after the index stroke classified by treatment group. Secondary measures include the total number of stroke patients with newly detected AF and their subsequent treatments. Final data collection for the primary outcome is due in late 2016.
Prof Kirchhof went on to discuss the association between AHREs and stroke. AHREs are defined as the duration of mode switch in a 24-hour period recorded on a cardiac resynchronization device. In some devices, mode switching occurs when 5 of 8 consecutive atrial beats are > 180 bpm; in others, the criteria for onset are 36 of 48 atrial cycles with a rate > 180 beats per minute [Shamnugan M et al. Europace. 2011]. He reviewed data from the ASSERT trial [Healey JS et al. N Engl J Med. 2012] showing that over 2.5 years, AHREs without AF occurred frequently in patients with pacemakers. Patients with AHREs but without clinical AF were significantly more likely to develop ischemic stroke or systemic embolism (HR, 2.49; 95% CI, 1.28 to 4.85; P = .007) and AF (HR, 5.56; 95% CI, 3.78 to 8.17; P < .001) compared with patients without AHREs.
In addressing the question regarding whether patients who experience AHREs should be anticoagulated, Prof Kirchhof recommended that clinicians first document AF by activating the storage capability of the pacemaker or by putting in loop recorders or noninvasive Holter monitors. If these methods suggest AF, then patients might be managed according to the algorithm published by the 3rd Atrial Fibrillation Competence NETwork/European Heart Rhythm Association Consensus Conference [Kirchhof P et al. Thromb Haemostat. 2011]. This could include the initiation of antithrombotic therapy and a review of the AHREs to determine whether patients’ symptoms are related to AHREs or AF and to adapt rate and rhythm control therapy.
Because the process of documenting AF vs AHREs is cumbersome and may leave the patient unprotected in the event that AF is present, Prof Kirchhof called for more trials of OACs in patients with AHREs, one of which is currently underway.
The ARTESIA trial [NCT01938248] is examining whether, when compared with aspirin, treatment with apixaban will reduce the risk of ischemic stroke and systemic emboli in patients with device-detected AHREs and no known AF.
Marcio Figueiredo, MD, State University of Campinas, Campinas, Brazil, presented a global overview of AF anticoagulation registries. He reviewed data suggesting that the prevalence of AF in the United States would likely increase more than 2-fold from 1995 to 2050—especially in people aged ≥ 75 years [Go AS et al. JAMA. 2001]. Current estimates are that in 2010, 33.5 million people likely experienced AF worldwide [Chugh SS et al. Circulation. 2014]. According to 1 estimate, hospitalizations among people with AF increased by 23% between 2000 and 2010; adjusted costs significantly rose from $6410 in 2000 to $8439 in 2010 (P < .001) [Patel NJ et al. Circulation. 2014].
Prof Figueiredo then discussed the importance of patient AF registries, in that they provide details about “real world” patients and allow researchers to recognize and evaluate the efficacy and safety of new therapies and changes over time. Registries also allow researchers to validate clinical classification schemes and to identify various pathophysiologies of AF among patients and countries. Prof Figueiredo highlighted RECALL—the Brazilian registry of AF that was established to document demographic data, antiplatelet and antithrombotic therapy, level of international normalized ratio control, warfarin discontinuation rates, and clinical outcomes. The target size is 5000 patients, and patients will be followed up for 12 months after the initial event. As of May 2015, 2024 patients have been recruited from all over Brazil.
According to Prof Figueiredo, RECALL data from 1902 patients show equal representation of men and women with the largest proportion of patients falling between the ages of 60 and 80 years. More than 3 in 4 patients have hypertension; 1 in 3 has heart failure; and 1 in 5 has diabetes (Table 1).
Percentage of Risk Factors Among Patients in the RECALL Registry
The majority of patients are taking the antiarrhythmic amiodarone at baseline; among those on anticoagulants, 61.2% are using warfarin, compared with 37% taking dabigatran, rivaroxaban, or apixaban. At 6 months of follow-up, 141 of 1185 (11.9%) patients had clinical events ranging from death (6.5%) to stroke (2.7%), major bleeding (2.3%), and systemic embolism (0.7%). Reasons that high-risk patients are not taking anticoagulants include patient preference, difficulty in performing international normalized ratio, and the perception of a high risk for bleeding. Prof Figueiredo noted that because these perceptions are common throughout Brazil, he would like to see strategies developed over time that will help change clinicians’ views on anticoagulation.
Eduardo Saad, MD, PhD, FHRS, Hospital Prό-Cardiaíco, Rio de Janeiro, Brazil, reviewed the use of anticoagulants following LAA closure or atrial ablation. While guidelines now recommend anticoagulation for the majority of patients with AF [Camm AJ et al. Eur Heart J. 2012], it is important to remember that anticoagulants have both benefits and risks.
While there are notable advantages to non–vitamin K antagonist OACs as compared with vitamin K antagonist therapy, including a lower risk of intracranial hemorrhage, Prof Saad also reviewed some disadvantages, such as short half-life, difficulties in titrating the dose, lack of an antidote, and a high cost.
Prof Saad then discussed the controversy as to whether OACs can be discontinued following a successful AF ablation. One review recommended that the decision regarding OACs following ablation should be based on patients’ baseline clinical risk scores rather than recurrence rates [Chao TF et al. J Thorac Dis. 2015]. Some data suggest that when high-risk patients discontinued OAC following ablation, thromboembolic events (TEEs) were more frequent [Nührich JM et al. Clin Res Cardiol. 2015]. However, a nonrandomized study of > 3300 patients showed that the risk-benefit ratio favored suspending OAC following successful ablation, even in patients at moderate to high risk of TEEs [Themistoclakis S et al. J Am Coll Cardiol. 2010]. In a much smaller study of 327 patients, no significant TEE-related morbidity was seen following ablation when patients discontinued OAC and antiarrhythmic drugs [Saad EB et al. Circ Arrhythm Electrophysiol. 2011].
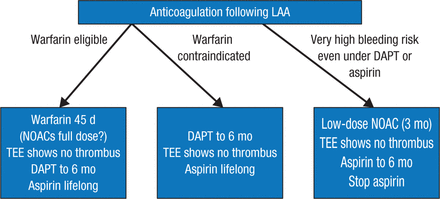
After discussing the benefits of first- and second-generation devices used to close the LAA, Prof Saad summarized current options for deciding whether to prescribe anticoagulation following LAA closure (Figure 1).
Current Options for Anticoagulation Following LAA Closure
- © 2015 SAGE Publications