Summary
Recent large registry studies of patients with AF have found an increased use of oral anticoagulation for prevention of thromboembolic events, consistent with the most recent Professional Guidelines. Although AF management has improved in clinical practice, anticoagulation use is often inappropriate and mortality rates remain high.
- atrial fibrillation
- bleeding
- EORP-AF
- GARFIELD-AF
- mortality
- NOAC
- OAC
- oral anticoagulation
- ORBIT-AF
- PREFER
- stroke
- thromboembolic events
- cardiology & cardiovascular medicine clinical trials
- cerebrovascular disease
- thrombotic disorders
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia and is associated with a heightened risk of morbidity and mortality. Many patients with occult AF are not diagnosed until they present with their first stroke. Most clinical guidelines recommend anticoagulation therapy for patients with AF except those at low risk for thromboembolic events [Jones C et al. BMJ. 2014; Camm AJ et al. Eur Heart J. 2012; Skanes AC et al. Can J Cardiol. 2012; Fuster V et al. Circulation. 2011]. The new European Society of Cardiology (ESC) guidelines suggest that treatment patterns have changed since the ESC EuroHeart survey was conducted a decade ago.
Laurent Fauchier, MD, Universite François Rabelais, Tours, France, discussed the EORP-AF General Registry, conducted to gain insights into current management of patients with AF [Lip GYH et al. Europace. 2014]. A total of 3049 patients from 9 countries were assessed at baseline and once a year for 3 years.
At baseline, common comorbidities included hypertension, coronary artery disease, and heart failure (HF). Oral anticoagulants (OACs) were used in > 78% of patients, most commonly vitamin K antagonists (VKAs; 71.6%). Other antithrombotics, mostly antiplatelet therapy with aspirin, were used in one-third of patients, and no antithrombotics in 4.8% of patients. The most common antiarrhythmic agent was amiodarone (21.5%). Rate control agents included β-blockers (69.2%) and digoxin (19.4%). OACs were used in 66.7% of patients with a CHA2DS2-VASc score of 9, with mostly antiplatelets in 33.3%.
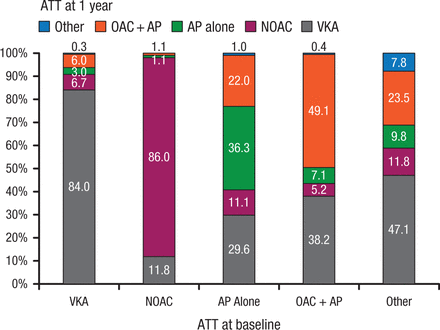
At 1 year, 84% of patients on VKAs at baseline remained on VKAs (Figure 1) [Lip GYH et al. Eur Heart J. 2014]. Among patients on non-VKA OACs (NOACs) at baseline, 86% remained on NOACs, 11.8% had switched to a VKA, and 1.1% to antiplatelet therapy alone. By 1 year, interventions included cardioversion (electrical, 9.7%; pharmacologic, 5.1%) and catheter ablation (4.4%).
Antithrombotic Use at 1 Year Based on Initial Regimen
Antithrombotic therapy use at 1 year based on initial/baseline antithrombotic regimen.
ATT, antithrombotic therapy; VKA, vitamin K antagonist; AP, antiplatelet therapy (most commonly aspirin); OAC, oral anticoagulant therapy.
Reprinted from Lip GY et al, Prognosis and treatment of atrial fibrillation patients by European cardiologists: One Year Follow-up of the EURObservational Research Programme-Atrial Fibrillation General Registry Pilot Phase (EORP-AF Pilot registry), Eur Heart J, 2014; Vol 35, Issue 47, Pages 3365-3376, by permission of Oxford University Press.
At 1 year, 5.7% of the patients had died, with 57.4% attributable to cardiac causes, most commonly HF (77.3%). One-year mortality was highest among asymptomatic vs symptomatic patients (9.4% vs 4.2%; P < .0001) [Boriani G et al. Am J Med. 2015]. Appropriate OAC use was less frequent among asymptomatic patients.
The PREFER registry collected data from patients with AF in 7 European countries [Kirchhof P et al. Europace. 2014]. Luis M. Rincón, Hospital Universitario Ramón y Cajal, Madrid, Spain, presented the data analysis from 7243 patients enrolled between January 2012 and January 2013. The patients were assessed at baseline and 1 year later.
The baseline analysis found a high rate of risk factors for stroke. The mean CHA2DS2-VASc score was 3.4 ± 1.8 and was ≥ 2 points in 84% of patients. At baseline, 86% of patients with a CHA2DS2-VASc score ≥ 2 and 70% of those with a CHA2DS2-VASc score of 1 had received OAC therapy. A comparison of antithrombotic treatment at baseline and follow-up showed that most eligible patients received OACs [Rincon LM et al. ESC 2014 (poster P6266)].
Patients treated with NOACs were more likely to have paroxysmal AF than those treated with VKAs (43.0% vs 23.5%; P < .01) [Rincon LM et al. ESC 2013 (poster P5138)]. Patients treated with VKAs more often had long-standing persistent or permanent AF than those treated with NOACs (45.2% vs 30.3%; P < .01). Patients receiving NOACs were younger than those treated with VKAs (70.3 ± 10.48 vs 72.1 ± 10.12 years; P < .01). The mean CHA2DS2-VASc and HAS-BLED scores were comparable between patients receiving NOACs and VKAs. For patients on VKAs, 24.2% were not within the therapeutic treatment range (TTR) according to the last 3 international normalized ratio (INR) measurements. Physicians tended to overestimate the level of INR control as only 17.3% were considered to have TTR out of range when questioned.
The goal of the ORBIT-AF registry was to characterize the treatment and outcomes of patients with AF in the United States [Piccini JP et al. Am Heart J. 2011]. About 10 000 patients from about 200 sites were enrolled, most before the availability of NOACs. The main outcomes were stroke or systemic embolism (SE), major bleeding, and other major adverse cardiovascular events, with a 3-year follow-up. Benjamin A. Steinberg, MD, Duke University Medical Center, Durham, North Carolina, USA, highlighted new data on heart rate, anticoagulation, bleeding risk, management of concurrent antithrombotics, and anticoagulation interruptions.
At baseline, 30% of patients had a resting heart rate of > 80 bpm, and heart rate increased with increasing symptom severity [Steinberg BA et al. ACC. 2015 (poster 247)]. Adjusted data based on time-variable covariates showed a lower risk of all-cause and cardiovascular death at heart rates of about 60 to 70 bpm, with increased risk at lower and higher rates.
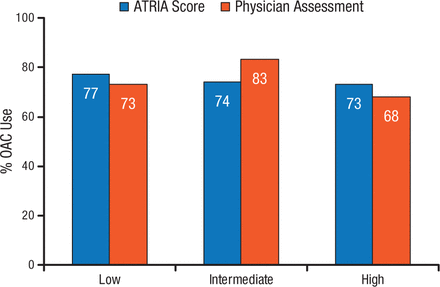
At least 1 contraindication to anticoagulation was present in 13% of patients, with the most common contraindications of prior bleeding (28%), patient refusal (28%), high bleeding risk (18%), and frequent falls or frailty (18%) [O’Brien EC et al. Am Heart J. 2014]. Mean CHADS2 scores were higher among patients with contraindications to anticoagulation (2.53 vs 2.22 without contraindications). Comparison of ATRIA bleeding risk scores with subjective physician bleeding assessment showed 63% physician agreement with ATRIA ≤ 3, 33% agreement with ATRIA = 4, and 13% agreement with ATRIA ≥ 5 [Steinberg BA et al. Circulation. 2014]. Anticoagulation use by bleeding risk according to ATRIA score and physician assessment is shown in Figure 2.
ORBIT-AF Registry: Anticoagulation According to Bleeding Risk
ATRIA indicates Anticoagulation and Risk Factors in Atrial Fibrillation; CHADS2, congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, and previous stroke or transient ischemic attack; and OAC, oral anticoagulation.
Reprinted from Steinberg BA et al. Lack of concordance between empirical scores and physician assessments of stroke and bleeding risk in atrial fibrillation: results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry, Circulation, 2014, Vol 129, Issue 20, Pages 2005-12, with permission from American Heart Association, Inc.
Among patients prescribed OACs plus aspirin, 39% did not have vascular disease [Steinberg BA et al. Circulation. 2013]. These patients had a significantly higher risk than those on OACs alone for major bleeding (HR, 1.53; 95% CI, 1.20 to 1.96; P = .0006).
Bridging anticoagulation was used in 24% of patients requiring OAC interruption for procedures and was associated with a higher risk for bleeding and cardiovascular events [Steinberg BA et al. Circulation. 2014].
The GARFIELD-AF registry [NCT01090362] is a large ongoing international prospective study of risk and outcomes in patients with newly diagnosed nonvalvular AF and ≥ 1 additional stroke risk factor. The study consists of 5 sequential cohorts, with the first beginning enrollment in 2010 and cohort 5 starting in 2015. A total of 42 536 patients have been enrolled. John Camm, MD, St George’s University, London, United Kingdom, presented the 1-year results from cohorts 1 and 2 (n = 17 168).
The 1-year event rates per person per year were mortality at 4.06%, stroke or SE at 1.37%, and major bleeding at 0.85%.
Factors associated with increased risk of death, stroke, or SE in the first year were age ≥ 75 years, current smoking, no anticoagulant therapy, renal failure, cardiac failure, diabetes, and time to therapeutic range < 60%. Current or previous hypertension, type of AF, and previous stroke or SE did not appear to increase risk. There was a trend for CHA2DS2-VASc and HAS-BLED risk scores to predict death, stroke or SE, and major bleeding events.
Patients who did not receive anticoagulation therapy had a higher risk of death (HR, 1.55; 95% CI, 1.32 to 1.81) and stroke or SE (HR, 1.71; 95% CI, 1.31 to 2.24) and a lower risk of major bleeding at 1 year (HR, 0.44; 95% CI, 0.29 to 0.67).
Johannes Brachmann, Klinikum Coburg, Coburg, Germany, focused on the detection of AF among stroke survivors. The risk of stroke can be decreased with anticoagulation therapy, but AF often is asymptomatic and only diagnosed after an index stroke [Keach JW et al. Heart. 2015]. Clinical risk scores for identifying stroke patients at risk for AF have demonstrated high sensitivity and specificity in single studies [Fujii S et al. J Neurol Sci. 2013; Suissa L et al. Stroke. 2009] but have not been prospectively studied in large populations.
In a 2004 study of patients with acute stroke or transient ischemic attack (TIA), AF was diagnosed in 2.7% after 1 electrocardiogram (ECG), 11.5% after multiple ECGs, and 1.4% after 24-hour Holter ECG. Another study reported a 5-fold increase in AF detection after stroke or TIA with 30-day ECG vs short-duration ECG [Gladstone DJ et al. N Engl J Med. 2014]. In the SURPRISE study, asymptomatic AF was detected by implantable loop recorder in 16.1% of patients at an average of 109 days after cryptogenic stroke [Christensen LM et al. Eur J Neurol. 2014].
Long-term monitoring with an implantable cardiac monitor (ICM) was compared with conventional monitoring after cryptogenic stroke in 441 patients [Sanna T et al. N Engl J Med. 2014]. The ICM significantly improved AF detection after 6 months (HR, 6.4; 95% CI, 1.9 to 21.7; P < .001), 12 months (HR, 7.3; 95% CI, 2.6 to 20.8; P < .001), and 36 months (HR, 8.8; 95% CI, 3.5 to 22.2; P < .001).
The evidence indicates that cryptogenic stroke is frequently associated with a time-dependent high rate of AF detection. The high AF rate in this population led to a switch to OACs for most patients. Patients with cryptogenic stroke should be monitored with long-term continuous ECG by an insertable cardiac monitor.
These recent studies of patients with AF reported that OAC use is high and has increased since implementation of the latest ESC guidelines. Despite this increase, 1-year mortality and morbidity remain high. Inappropriate use of OACs for patients with few or no risk factors is common, and assessment of contraindications to OACs is a challenge. VKAs remain the most commonly used antithrombotic therapy, but INR control is often inadequate. Antiplatelet agents are widely used, but often inappropriately. NOAC use is most common among younger patients but is still low. Concomitant use of aspirin with OACs is associated with increased bleeding risk and no clear evidence of benefit.
- © 2015 SAGE Publications