Summary
Two new implantable cardioverter defibrillator systems developed for compatibility with magnetic resonance imaging demonstrate safety and efficacy in the Evera MRI and ProMRI trials, with no significant changes in ventricular pacing threshold < 5.0 V or ventricular sensing. In addition, there were no serious device-related events in both studies.
- implantable cardioverter defibrillators
- ICD
- ventricular pacing
- MRI
- Evera MRI
- ProMRI
- NCT02117414
- NCT02096692
- cardiology & cardiovascular medicine clinical trials
- imaging modalities
- interventional techniques & devices
Two new implantable cardioverter defibrillators (ICDs) have been demonstrated to be safe and effective in magnetic resonance imaging (MRI) scanning conditions. Michael R. Gold, MD, PhD, Medical University of South Carolina, Charleston, South Carolina, USA, presented data from the Evera MRI study [Gold MR et al. J Am Coll Cardiol. 2015], and Khaled A. Awad, MD, University of Alabama Birmingham School of Medicine, Birmingham, Alabama, USA, presented data from the ProMRI study [NCT02096692].
Due to the risks associated with devices during imaging, MRI is contraindicated in many patients with ICDs. Although MRI-safe pacemakers are available, there are currently no MRI-safe ICDs approved by the FDA. MRI has been used in some ICD patients in select centers; however, limitations must be imposed, such as restriction of imaging to particular body areas. The purpose of the Evera MRI and ProMRI studies was to evaluate the safety and efficacy of new ICD systems that were developed to be MRI compatible.
In the international open-label prospective Evera MRI study, 263 patients who had successful ICD implantation were randomly assigned 2:1 to undergo MRI scans of the chest, spine, and head or to enter a waiting period. Patients in the MRI arm received clinically relevant scans with a maximized gradient slew rate, a whole body < 2.0 W/kg with 20 minutes of active scan duration, and 50 minutes in the MRI bore.
The primary efficacy end points were ventricular pacing capture threshold (> 0.5 V) and ventricular sensing (> 50% decrease). The primary safety end points included complications related to the MRI, loss of capture, and treated ventricular arrhythmias during the scan. Success was defined as achievement of all 3 end points. Patients were excluded if they required device upgrades, change-outs, lead extractions, or lead or device revisions or if they had abandoned or capped leads or a non-MRI-compatible device or material implant.
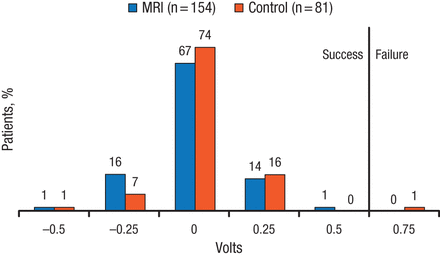
The change in ventricular pacing capture threshold was noninferior between the 2 arms, with 100% and 98.8% success rates in the MRI and control arms, respectively (PNoninferiority < .0001; Figure 1). In addition, there was no difference in ventricular sensing amplitude at 1 month post-MRI compared with patients who underwent a waiting period, with success achieved by 99.3% and 98.8% in the MRI and control arms, respectively (PNoninferiority = .0001). The MRI arm achieved noninferiority for the secondary end points, including atrial pacing capture threshold, atrial sensing amplitude, right ventricular defibrillation impedance, and system-related complication-free rate > 80%.
Change in Ventricular Pacing Capture Threshold in the Evera MRI Study
MRI, magnetic resonance imaging.
Reproduced with permission from MR Gold, MD, PhD.
The complication-free rate was 100% among all 147 patients who underwent MRI; however, 2 patients experienced implant site warmth, and 1 patient each experienced atrial tachycardia, back pain, and burning sensation of the forehead.
In the multicenter prospective nonrandomized single-arm ProMRI study, 154 patients received the Iforia ProMRI ICD system and underwent a thoracic spine or cardiac 1.5T closed-bore MRI. In addition, the maximum slew rate was ≤ 200 T/ms per axis, and the specific absorption rate was up to 2.0 W/kg for the entire body and 3.2 W/kg for the head. Patients were excluded from the study if they had planned cardiac surgery within 3 months, had a life expectancy < 3 months, had an abandoned ICD or pacemaker leads, were pregnant, or had any prostheses or devices that were non-MRI compatible. The end points of the ProMRI study included freedom from the following: serious adverse device events, ventricular capture threshold increase, and decrease in ventricular sensing.
Freedom from ventricular capture threshold > 0.5 V was achieved by 100% of patients (n = 153), with most patients experiencing no change in voltage (P < .001). Similarly, the freedom from decrease in ventricular sensing was achieved by 99.3% of patients at 1 month (P < .001). In addition, there were no significant changes in P wave amplitude, atrial pacing threshold, right ventricular pacing impedance, shock impedance, and batter capacity after MRI. In the study, 43 adverse events occurred that were deemed not related to the device; therefore, there were no serious adverse device events.
In conclusion, Dr Gold and Dr Awad indicated that the data from the Evera MRI and ProMRI studies demonstrated that the Evera MRI and Iforia ICD systems, respectively, were safe and effective in patients undergoing MRI, with no difference in pacing or sensing detected.
- © 2015 SAGE Publications