Summary
This article provides updates in the fields of device therapy for ventricular arrhythmias—including risk stratification regarding patient selection for implantable cardioverter defibrillators, the optimal programming for implantable cardioverter defibrillators to reduce inappropriate shocks, the impact of sports participation on patients with implantable cardioverter defibrillators, and the future of resynchronization therapy.
- sudden cardiac death
- myocardial infarction
- ventricular tachycardia
- risk stratification
- implantable cardioverter defibrillator
- ICD programming
- antitachycardia pacing
- sports
- cardiac resynchronization therapy
- interventional techniques & devices
During this state-of-the-art session, experts discussed aspects of managing patients with ventricular arrhythmias. David S. Cannom, MD, University of California, Los Angeles, David Geffen School of Medicine, Los Angeles, California, USA, opened the session with an update on risk stratification for sudden cardiac death.
It was determined 30 years ago that a left ventricular ejection fraction (LVEF) < 40% was indicative of increased mortality risk. However, as one 2005 study showed, not much progress has been made; once LVEF is elevated > 45%, it does not add much new information to help assess cardiovascular risk in heart failure patients. As a sole risk stratification tool, it misses the majority of sudden cardiac death cases that occur in individuals with preserved or only moderately reduced LVEF. According to Dr Cannom, even among patients with severely reduced LVEF, only a portion will benefit from the implantable cardioverter defibrillator (ICD), since some still have a low individual risk.
Daubert and colleagues used data from the MADIT II electrophysiology study to show that inducibility predicted an increased likelihood of ventricular tachycardia (VT) and that noninducible patients had more risk factors as well as higher VT events and mortality rates [Daubert JP et al. J Am Coll Cardiol. 2006]. Unfortunately, electrophysiology studies have poor reproducibility.
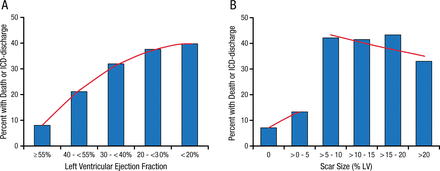
There have been advances, however. One study showed that myocardial scarring, detected by magnetic resonance imaging, is useful in stratifying risk when selecting patients for an ICD [Klem I et al. J Am Coll Cardiol. 2012]. In patients with LVEF > 30%, significant scarring (> 5% left ventricular [LV]) identifies a high-risk cohort similar in risk to those with LVEF ≤ 30% (P = .56). Conversely, in patients with LVEF ≤ 30%, minimal or no scarring identifies a low-risk cohort similar to those with LVEF > 30% (P = .71; Figure 1).
Myocardial Scarring as Predictor of Death or ICD Discharge
The percentage of patients with the primary endpoint of death or appropriate ICD discharge is shown according to different levels of left ventricular ejection fraction (Panel a) and scar size (Panel b). For ejection fraction, the trendline (red line) shows a positive slope over the entire range, indicating that event rate monotonically increases with decreasing LVEF. In contrast, a marked step-up in event rate is noted for scar size greater than 5% of left ventricular mass, which however does not rise further with increasing scar size.
Reprinted from J Am Coll Cardiol, Vol. 60, Klem I et al., Assessment of Myocardial Scarring Improves Risk Stratification in Patients Evaluated for Cardiac Defibrillator Implantation, Pages No. 408-420, Copyright (2012), with permission from American College of Cardiology Foundation.
Optimal ICD Programming
The most important objective of optimal ICD programming is the detection and treatment of life-threatening VT/ventricular fibrillation (VF). The reduction of unnecessary shocks is an important secondary objective. Charles D. Swerdlow, MD, Cedars-Sinai Medical Center, Los Angeles, California, USA, provided a preview of a few of the recommendations from the forthcoming (fall 2015) expert consensus statement on ICD programming and testing, led by several global heart rhythm societies.
Two draft recommendations for supraventricular tachycardia (SVT)–VT discrimination algorithms are expected. The primary recommendation is to program SVT-VT discrimination with recommended rates ≤ 200 to 230 beats per minute (bpm), unless contraindicated (class I: programming should be done). The secondary recommendation, which is for subcutaneous ICD, calls for programming 2 tachycardia zones with SVT-VT discrimination ≤ 200 to 230 bpm (class IIA: programming is reasonable). Dr Swerdlow believes that the most useful individual discriminator is the ventricular electrogram morphology recorded from the shock (or far-field channel).
Optimal ICD programming to reduce unnecessary shocks should minimize inappropriate detection of SVT as VT, oversensing of rapid intervals as VT/VF intervals, and unnecessary therapy for slow or self-terminating VT. It should also terminate life-threatening VT with programmed antitachycardia pacing (ATP) when possible and prevent proarrhythmia. To that end, the draft consensus includes recommendations that the following should be programmed: a lead failure alert based on oversensing, with or without abrupt impendence changes (class I); lead “noise” algorithms that withhold shocks when the VT/VF on the sensing channel is not confirmed on the far-field channel (class IIb: programming may be considered); and algorithms designed to reject T-wave over-sensing (class IIB). The consensus statement is also expected to include a recommendation to program ATP for all VT/VF zones ≤ 230 bpm in patients with structural heart disease and to program burst ATP in preference to ramp ATP (both class I).
In summary, the draft recommendations include longer duration (6-12 seconds or 30 intervals) and faster rates (185-200 bpm) for primary VT/VF detection, SVT-VT discriminators with recommended values (≤ 200 to 230 bpm), the use of far-field electrogram morphology discriminators if available, and the use of ATP burst ≤ 230 bpm in patients with structural disease.
Impact of Sports Participation on Patients With ICDs
Rachel J. Lampert, MD, Yale School of Medicine, New Haven, Connecticut, USA, reported that many athletes with ICDs can participate in sports and recommended that sports participation for these athletes should be an individualized decision.
Dr Lampert reported results from a prospective multinational registry in individuals with ICDs who were participating in organized or high-risk sports [Lampert R et al. Circulation. 2015]. The most common diagnoses were long QT syndrome, hypertrophic cardiomyopathy, and arrhythmogenic right ventricular cardiomyopathy. There were no incidents of tachyarrhythmic death or externally resuscitated tachyarrhythmia during or after sports nor injury due to arrhythmia or shock during sports at ≥ 2 years (95% CI, 0% to 1.5%). Appropriate shocks were more common during physical activity, competition, and other activity than at rest (Table 1). Lead malfunction rates were similar to those reported in unselected populations.
Shock Activity During Sports
Dr Lampert noted several caveats to the data. There were few athletes participating in violent contact sports, such as football or hockey, and there were no players with subcutaneous ICDs. Dr Lampert recommended stress testing to evaluate individual risk, as well as optimum programming for preventing unnecessary shocks, monitoring, and possible use of β-blockers.
Future of Cardiac Resynchronization Therapy
The rates for cardiac resynchronization therapy (CRT) nonresponse have remained stable at about 30% over the past 8 years [Gorcsan John III. Circulation. 2011]. The nonresponse rate, however, is dependent on the definition/measurement of the end point, as well as time. Kenneth A. Ellenbogen, MD, Virginia Commonwealth University, Richmond, Virginia, USA, discussed individualizing and optimizing responses to CRT as ways to address this issue.
In one study, Q LV duration was associated with CRT response, indicating that Q LV measurement may be useful to guide LV lead placement [Gold MR et al. Eur Heart J. 2011]. The efficacy of CRT depends strongly on the patient-specific electrophysiologic substrate. Jia and colleagues suggested that electrocardiographic imaging, followed by the use of an endocardial (vs epicardial) LV pacing electrode, may be a better way of measuring this [Jia P et al. Heart Rhythm. 2006].
One pacing approach employs ultrasound-mediated pacing that uses an ultrasound transmitter to bring acoustic waves from the chest wall to a receiver electrode in contact with the myocardium, which then converts the ultrasound energy to electrical energy adequate to pace.
Another approach is direct His bundle pacing, which produces synchronous ventricular depolarization and improved cardiac function relative to apical pacing. Long-term direct His bundle pacing results in a reduction of LV dimensions and improved cardiac function [Barba-Pichardo R et al. Europace. 2013].
Endocardial LV pacing and direct His bundle pacing are both being assessed as potential alternatives to epicardial pacing.
- © 2015 SAGE Publications