Summary
The TRIAGE study was conducted to determine whether platelet reactivity testing added to clinical assessment of patients treated with prasugrel vs clopidogrel would better identify patients at high ischemic risk. However, low enrollment resulted in a sample size too small to detect a between-group difference; none was found for any of the prespecified efficacy or safety outcomes.
- clopidogrel
- high on-treatment platelet reactivity
- HTPR
- ischemic risk
- low on-treatment platelet reactivity
- LTPR
- prasugrel
- TRIAGE
- cardiology & cardiovascular medicine clinical trials
Jaya Chandrasekhar, MBBS, Icahn School of Medicine at Mount Sinai, New York, New York, USA, presented results of the TRIAGE study [NCT01582217], demonstrating that patients with high on-treatment platelet reactivity (HTPR) receiving prasugrel and patients with low on-treatment platelet reactivity (LTPR) treated with clopidogrel had similar ischemic and bleeding outcomes.
HTPR in patients treated with clopidogrel is associated with a greater incidence of adverse cardiac events such as stent thrombosis, myocardial infarction (MI), and even death. However, testing platelet function prior to thienopyridine selection in patients undergoing percutaneous coronary intervention (PCI) has not been shown to correlate with improved outcomes in recent randomized trials, and the role of screening for platelet function in the context of ischemic and bleeding risks has not been investigated.
TRIAGE was a multicenter, prospective, observational study. The objective was to compare outcomes in patients treated with prasugrel vs clopidogrel at PCI following determination of platelet reactivity in conjunction with clinical risks. The primary safety and efficacy end points were the rate of major adverse cardiac events (MACE) and the rate of BARC type 2, 3, or 5 bleeding, respectively, at 1-year follow-up.
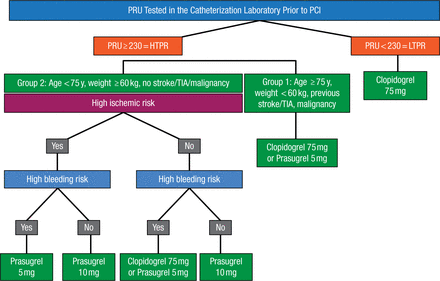
Platelet reactivity was tested immediately prior to PCI, and HTPR was defined as P2Y12 Reaction Units (PRU) ≥ 230. Patients were further split into treatment groups based on their ischemic and bleeding risk levels (Figure 1). High ischemic risk was defined as undergoing PCI for acute coronary syndromes or stent thrombosis, high angiographic risk PCI, or 30-day stent thrombosis score of ≥ 6. High bleeding risk was defined as having a bleeding risk score ≥ 10, recent surgery, recent bleeding history, or bleeding diathesis.
TRIAGE Study Algorithm
HTPR, high on-treatment platelet reactivity; LTPR, low on-treatment platelet reactivity; PCI, percutaneous coronary intervention; PRU, P2Y12 Reaction Units; TIA, transient ischemic attack.
Reproduced with permission from J Chandrasekhar, MBBS.
The anticipated study sample size was 1000 patients, but recruitment was terminated due to slow enrollment at 318 patients (mean age, 65.9 years; 19.0% women). Based on the study criteria, 40% of patients were classified to be at high ischemic risk, 58% with PRU ≥ 230 and/or high ischemic risk, and 34% at high bleeding risk. Clopidogrel was continued in 72% of patients, whereas 28% received prasugrel.
The primary efficacy end point did not differ significantly between treatment groups: MACE (death, nonfatal MI, or stent thrombosis) occurred in 3.5% of patients on clopidogrel and 4.4% on prasugrel (P = .70). The rate of secondary ischemic end points including periprocedural MI was also similar between the treatment groups with nonsignificant P values. The primary safety end point occurred in 7.9% of patients on clopidogrel and 5.6% on prasugrel (P = .47). The secondary bleeding end points were also nonsignificant, with patients on clopidogrel experiencing a numerically but not statistically higher rate of bleeding events.
MACE and secondary ischemic end points occurred at a similar rate in patients with PRU < 230 and those with PRU ≥ 230. Analogously, when patients with PRU < 208 were compared with those with PRU ≥ 208, ischemic end points were numerically but nonsignificantly different (P = .08).
In conclusion, the TRIAGE study did not find a significant difference between groups for any of the prespecified efficacy or safety end points. The low enrollment resulted in a sample size that was too small to detect any significant differences between groups. Other study limitations were the unblinded treatment, low event rate, and use of an unvalidated treatment algorithm, although it incorporated validated ischemic and bleeding score components. The difference in the number of ischemic and bleeding events between groups may suggest that platelet function testing may identify more patients at a high ischemic risk than clinical assessment alone, but this must be tested in a larger study.
- © 2015 SAGE Publications