Summary
Percutaneous coronary intervention is the primary treatment for patients with acute coronary syndrome. Selective use of manual thrombectomy may be an effective option. P2Y12 inhibitors are a treatment of choice in patients with STEMI. Bivalirudin reduces adverse cardiac events and is associated with reduced bleeding. When percutaneous coronary intervention is delayed in patients with STEMI, fibrinolytic therapy should be administered.
- acute coronary syndrome
- bivalirudin
- fibrinolytic therapy
- glycoprotein IIb/IIIa
- Impella
- intraaortic balloon pump
- P2Y12 inhibitors
- NSTEMI
- percutaneous coronary intervention
- pharmacoinvasive
- STEMI
- thrombin inhibitor
- pharmacology
- cardiology & cardiovascular medicine guidelines
- interventional techniques & devices
Approximately 683 000 patients were discharged from US hospitals with a diagnosis of acute coronary syndrome (ACS) in 2009 [O’Gara PT et al. Circulation. 2013]. Among patients with myocardial infarction (MI), 25% to 40% have STEMI. Stent thrombosis following percutaneous coronary intervention (PCI) is higher among patients with ACS, but mortality rates have declined with the prompt use of interventional and pharmacologic treatments.
Device Interventions for Multivessel Disease
Multivessel disease is present in approximately 50% of patients undergoing PCI for STEMI [Hsieh V, Mehta SR. Curr Treat Options Cardiovasc Med. 2013]. These patients generally are older, have more risk factors, and have a worse short- and long-term prognosis. Guidelines discourage performing PCI on a nonculprit artery, especially during primary PCI (PPCI) [Windecker S et al. Eur Heart J. 2014; O’Gara PT et al. Circulation. 2013].
Evidence from newer trials suggests that more complete revascularization in patients with multivessel disease may be safer with current technology and antiplatelet therapy, according to Eric R. Bates, MD, University of Michigan Health System, Ann Arbor, Michigan, USA. A meta-analysis of culprit vs multivessel PCI supported revisiting the guideline recommendations [Zhang D et al. PloS One. 2014]. Of 4 randomized trials, 2 earlier trials showed little difference between groups, while 2 more recent trials demonstrated some benefit with multivessel PPCI (Table 1).
Randomized Clinical Trials of Culprit-Only vs MV PPCI
The limited evidence suggests that multivessel PCI is feasible and probably safe. PCI of nonculprit vessels should conform to elective PCI standards and is not appropriate for intermediate, chronic total occlusion, or complex lesions. Patients should be carefully selected and should have stable hemodynamics and normal renal function. There may be advantages to staging multivessel PCI rather than performing it during the initial procedure.
Device Interventions to Improve Myocardial Reperfusion
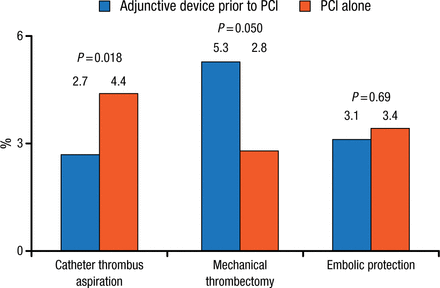
Impaired reperfusion after PPCI is associated with poor outcomes. Anthony A. Bavry, MD, University of Florida Health, Gainesville, Florida, USA, discussed studies of new technologies designed to improve myocardial reperfusion. An early meta-analysis of randomized trials reported that mortality was reduced with aspiration thrombectomy, increased with mechanical thrombectomy, and similar with embolic protection during acute MI vs PCI alone (Figure 1) [Bavry AA et al. Eur Heart J. 2008].
Six-Month Mortality: Adjunctive Devices Prior to PCI vs Conventional PCI
Reprinted from Bavry AA et al., Role of adjunctive thrombectomy and embolic protection devices in acute myocardial infarction: a comprehensive meta-analysis of randomized trials, Eur Heart J. 2008;29(24):2989-3001, by permission of European Society of Cardiology.
A more recent meta-analysis demonstrated similar overall rates of major adverse cardiac events, all-cause mortality, reinfarction, target vessel revascularization, and stroke with aspiration thrombectomy vs conventional PCI [Kumbhani DJ et al. J Am Coll Cardiol. 2013]. Other studies found no benefit of manual aspiration thrombectomy before PCI vs PCI alone [Jolly SS et al. N Engl J Med. 2015; Fröbert O et al. N Engl J Med. 2013]. Another study found no difference in thrombus size after manual thrombectomy vs PCI alone, but infarct size was significantly reduced by intralesional abciximab vs no abciximab (P = .03) [Stone GW et al. JAMA. 2012]. Sianos and colleagues [J Am Coll Cardiol. 2007] reported that patients with a large thrombotic burden treated with rheolytic thrombectomy had an infarct-related artery stent thrombosis rate of 0% vs 11.3% for patients without thrombectomy.
The evidence shows that current devices are not completely effective. Routine aspiration thrombectomy is no longer recommended. Not all patients with STEMI need thrombectomy; those who can derive the most benefit should be identified.
Device Interventions for Cardiogenic Shock
The mortality rate in patients with cardiogenic shock is close to 50% [Thiele H et al. Eur Heart J. 2015]. In patients with STEMI shock, early revascularization can improve late survival [Reynolds HR, Hochman JS. Circulation. 2008]. According to David A. Cox, MD, Lehigh Valley Health Network, Allentown, Pennsylvania, USA, the intra-aortic balloon pump (IABP) has been a standard treatment. However, there is no positive randomized evidence for the use of the IABP in patients with cardiogenic shock. The IABP-SHOCK II randomized trial found that patients receiving the IABP vs control patients had no reduction in mortality at 30 days (39.7% vs 41.3%) [Thiele H et al. ESC 2012] or at 1 year (52% vs 51%) [Thiele H et al. Lancet. 2013].
The Impella is a miniaturized pump for single femoral access that actively unloads the left ventricle. Although the early version of the Impella was complicated, the device is now as easy to insert as the IABP. The ISAR-SHOCK trial [Seyfarth M et al. J Am Coll Cardiol. 2008] found that the Impella was feasible and safe and provided superior hemodynamic support compared with IABP. The cardiac index was 0.49 with the Impella vs 0.11 with the IABP (P = .02). Overall 30-day mortality was 46% in both groups. The Impella also improved hemodynamic and metabolic parameters in the USpella trial [O’Neill WW et al. J Interv Cardiol. 2014]. Survival to discharge was superior in patients receiving the Impella before (65.1%) vs after (40.7%) the intervention (P = .003), although it was not significantly different compared with patients treated with the IABP.
Thus far, all IABP trial results have been negative. The Impella provides better cardiac output and unloading compared to the IABP. The ongoing randomized DanShock trial [NCT01633502] will provide more data.
P2Y12 Receptor Antagonists
Stent thrombosis occurs more frequently among patients with STEMI than among those with NSTEMI. Treatment of STEMI with the direct thrombin inhibitor bivalirudin is associated with a lower risk of major bleeding and reduced cardiac mortality compared with unfractionated heparin and glycoprotein IIb/IIIa inhibitors (GPIs) [Stone GW et al. J Am Coll Cardiol. 2014]. However, acute stent thrombosis is more common among patients receiving bivalirudin monotherapy. Thomas D. Stuckey, MD, Cone Health Heart and Vascular Center, Greensboro, North Carolina, USA, discussed the use of P2Y12 platelet inhibitors for patients with STEMI.
P2Y12 platelet inhibitors include clopidogrel, prasugrel, ticagrelor, and cangrelor, and they have been evaluated in many trials in patients with ACS (Table 2). The guidelines recommend early use of clopidogrel, prasugrel, or ticagrelor for patients with STEMI, but prasugrel should not be used for patients with STEMI and prior stroke or transient ischemic attack, those with low body weight (< 60 kg), or those aged > 75 years [O’Gara PT et al. J Am Coll Cardiol. 2013]. In patients with NSTEMI, ticagrelor and clopidogrel are preferred treatments [Amsterdam EA et al. J Am Coll Cardiol. 2014].
Trials of P2Y12 Inhibitors in Patients With ACS
Dr Stuckey concluded that potent antiplatelet therapies such as ticagrelor and prasugrel work well during periods of heightened platelet activity and should be the treatment of choice in patients with STEMI without high bleeding risk. Platelet inhibition onset with oral agents is delayed in patients with STEMI. This delay may be overcome with pre-hospital P2Y12 inhibitors, crushed ticagrelor, intravenous bolus GPIs, or cangrelor. New, adequately powered randomized trials and registries are needed to address the interactions between antiplatelet and antithrombin strategies to optimize the tradeoffs between ischemic outcomes, bleeding, mortality, and cost.
Thrombin Inhibitors and GPIs
The treatment of ACS has evolved from aspirin and heparin therapy to a bleeding avoidance strategy with bivalirudin. However, 1 study with contradictory results has impeded this progress. Roxana Mehran, MD, Icahn School of Medicine at Mount Sinai, New York, New York, USA, reviewed the evidence in order to clarify uncertainties.
Multiple studies demonstrated the effectiveness of GPIs when added to heparin for preventing periprocedural ischemic complications with PCI. GPIs enhance vessel patency and reduce ischemic complications when added to thrombolytic therapy in patients with STEMI. Heparins have several limitations, including unpredictability, increased bleeding, and the need for dose increases.
Several large trials have found bivalirudin to be effective and safe in patients with ACS (Table 3).
Large-Scale Multicenter Randomized Trials in Patients With ACS
In the single-center study HEAT-PPCI [Shahzad A et al. Lancet. 2014], 1829 patients with STEMI scheduled for emergency angiography were randomly assigned to bivalirudin vs heparin. In contrast with the other bivalirudin studies, bivalirudin vs heparin was associated with higher rates of major adverse cardiac events (8.7% vs 5.7%; P = .01) and stent thrombosis (3.4% vs 0.9%) at 30 days.
More than 10 000 patients have participated in randomized trials of PPCI with bivalirudin. Bivalirudin has consistently reduced bleeding, increased early stent thrombosis, and reduced overall net adverse cardiac events, and it has been associated with a trend toward lower mortality. A larger prospective study of bivalirudin vs heparin monotherapy is needed, but at this time, Dr Mehran recommends bivalirudin as the antithrombotic agent of choice for PPCI.
Pharmacoinvasive Approach
Ramesh Daggubati, MD, East Carolina University, Greenville, North Carolina, USA, discussed the pharmacoinvasive approach for patients with STEMI. When PCI cannot be performed within 120 minutes of first medical contact, patients with STEMI and symptom onset within the previous 12 hours should be given fibrinolytic therapy [O’Gara PT et al. Circulation. 2013].
In the STREAM study [Armstrong PW et al. N Engl J Med. 2013], patients with STEMI who presented within 3 hours of symptom onset and were unable to undergo PPCI within 1 hour of medical contact were randomized to PPCI or fibrinolytic therapy with bolus tenecteplase, clopidogrel, and enoxaparin before undergoing rescue PCI. The median times from onset of symptoms to start of fibrinolysis or PPCI were 100 and 178 minutes, respectively. At 30 days, there was no significant difference in the primary end point (death, shock, congestive heart failure, or reinfarction) between the PPCI (14.3%) and fibrinolysis (12.4%) groups (P = .21). TIMI flow rates after PCI were similar in both groups. Stroke rates were low in both groups, but intracranial hemorrhagic and primary ischemic stroke rates were higher in the fibrinolysis group.
Dr Daggubati noted that transfer delays remain a challenge for patients with STEMI. Patients who present to a facility without a catheterization laboratory within 3 hours of symptom onset should be treated with fibrinolytic therapy if there is any doubt that the patient can get PPCI within the recommended timeframe.
Standard treatments for patients with ACS include interventional and pharmacologic therapies. Many uncertainties exist regarding the optimal treatment strategies for individual patients, but increasing evidence and new technologies are helping to guide treatment selection.
- © 2015 SAGE Publications