Summary
AVP-825 is a product containing a low-dose powder form of sumatriptan that can be delivered intranasally to treat migraines. It is better tolerated than higher doses of oral sumatriptan and has a faster onset of action. A pooled analysis of 2 studies confirmed that AVP-825 confers rapid headache relief sustained over 48 hours.
- migraine
- sumatriptan
- AVP-825
- powder intranasal delivery
- efficacy study
- safety study
- headache severity score
- headache pain relief
- neurology clinical trials
- interventional techniques & devices
High-dose (100 mg) sumatriptan tablets are commonly used to treat migraines; however, they have a relatively slow onset of action and may be poorly absorbed because of impaired gastrointestinal impairment occurring during migraine. This has led to the development of a low-dose (22 mg) sumatriptan powder delivered intranasally through a breath-powered delivery system (AVP-825). In a pharmacokinetic study, AVP-825 was shown to have fewer triptan-associated adverse events and have a faster onset of action compared with tablets [Obaidi M et al. Headache. 2013]. Results of pooled analysis of data from a phase 2 [Djupesland PG et al. Cephalalgia. 2010] and a phase 3 [TARGET; Cady RK et al. Headache. 2015] trial of AVP-825 were presented in a poster by Roger K. Cady, MD, Headache Care Center, Springfield, Missouri, USA, and showed that AVP-825 conferred rapid headache relief that was sustained over placebo out to 48 hours and was well tolerated.
Both studies were randomized, multicenter, double-blind, placebo-controlled, parallel-group trials that included patients with migraines, with headache severity scores of grade 2 or 3 for at least 1 year, and with no known resistance to sumatriptan. The objective was to evaluate the efficacy and safety of AVP-825 using a larger, uniform pool of patients. Outcomes included:
-
the proportion of patients with pain relief, freedom from pain, no clinical disability, and no migraine-associated symptoms (eg, nausea, vomiting, photophobia, and phonophobia); and
-
meaningful relief (subject-reported interpretation) within 120 minutes of treatment.
The percentage of patients requiring rescue medication over the first 48 hours after treatment, and the frequency and severity of treatment-emergent adverse events (TEAEs), were also recorded.
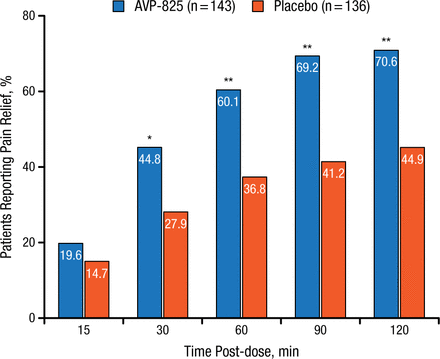
The pooled study included 279 patients randomized to either AVP-825 (n = 143) or placebo (n = 136). Significantly more patients receiving AVP-825 experienced pain relief (defined as reduction of their headache severity score from moderate or severe to mild or none) 30 to 120 minutes after treatment compared with those receiving placebo (P < .01; Figure 1).
Comparison of Pain Relief With AVP-825 and Placebo
*P < .01; **P < .001.
Reproduced with permission from RK Cady, MD.
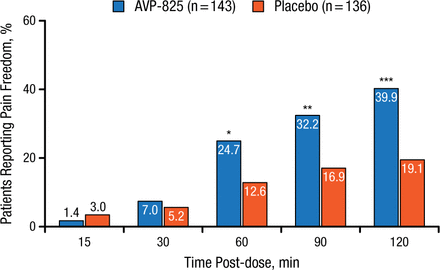
Similarly, a higher percentage of patients experienced relief from pain (defined as reduction of their headache severity score from mild, moderate, or severe to none) than those receiving placebo, significantly (P < .05) at 60, 90, and 120 minutes post-administration (Figure 2).
Comparison of Pain Freedom With AVP-825 and Placebo
*P < .05; **P < .01; ***P < .001.
Reproduced with permission from RK Cady, MD.
At 120 minutes, significantly more AVP-825–treated patients reported no clinical disability (P < .01), no migraine-associated symptoms (P < .05), and meaningful pain relief (P < .001). Additional rescue medication was less likely to be used during the first 48 hours by patients in the AVP-825 group. More AVP-825–treated patients reported TEAEs: 36% vs 11% for placebo. The most common TEAEs were abnormal taste, nasal discomfort, rhinorrhea, and rhinitis. There was a very low incidence of triptan sensations associated with AVP-825.
AVP-825 is a drug–device combination product containing low-dose (22 mg) sumatriptan delivered intranasally via a breath-powered device. As a treatment for migraines, it is well tolerated and delivers rapid headache relief that is sustained over placebo out to 48 hours.
- © 2015 SAGE Publications