Summary
This article addresses biomarkers in patients with migraine, the association of hypertension with recurrent intracerebral hemorrhage, and genetic research in autism. Regional cortical thickness measurements may be a useful biomarker for migraine. Inadequate blood pressure control increases risk of recurrent intracerebral hemorrhage. Genetic study of autism can be facilitated by identifying convergent molecular pathways.
- autism
- biomarker
- blood pressure
- convergence
- cortical thickness
- genetics
- intracerebral hemorrhage
- migraine
- neurology clinical trials
- neurology genomics
Practicing neurologists are confronted with many challenges in the diagnosis and treatment of neurologic disorders. Among the contemporary clinical issues explored at the American Academy of Neurology (AAN) 2015 Annual Meeting were the neurobiology of pain in adults with migraine, blood pressure (BP) control and the risk of recurrent intracerebral hemorrhage (ICH), and the development of targeted treatments for autism.
Cortical Thickness Measurements Promising as a Biomarker for Migraine
Migraine is typically diagnosed and subclassified according to the International Classification of Headache Disorders (ICHD) symptom-based criteria, which are derived from the opinions of an expert panel. The lack of an objective biomarker makes it difficult to test the validity of the ICHD criteria and optimize the diagnosis. Todd J. Schwedt, MD, Mayo Clinic, Phoenix, Arizona, USA, presented evidence showing that structural imaging might provide an objective biomarker for migraine.
Functional magnetic resonance imaging (MRI) in patients with migraine has demonstrated hyperexcitability of brain regions that facilitate sensory processing and atypical functional connectivity of sensory processing regions [Schwedt TJ et al. Lancet Neurol. 2015]. Structural MRI of cortical volume, surface, and thickness is a practical way to obtain data for a potential biomarker for migraine. A study in healthy participants found correlations between cortical thickness and pain sensitivity [Erpelding N et al. Pain. 2012]. Another study found that healthy controls had a significant negative correlation between cortical thickness and pain thresholds, while patients with migraine had a nonsignificant positive correlation [Schwedt TJ, Chong CD. PLoS One. 2014].
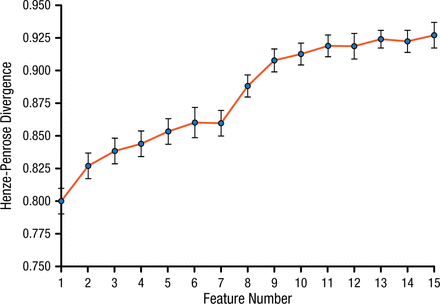
Dr Schwedt and colleagues conducted a study to identify the brain regions with interregional cortical thickness correlations that differed the most between patients with migraine (n = 64) and healthy controls (n = 39) [Schwedt TJ et al. PLoS One. 2015]. The participants underwent structural 3T MRI imaging. Cortical thickness was measured for 35 regions within each hemisphere, and cortical thickness correlations among these regions were identified. A multivariate model comprising 15 interregional cortical thickness correlations accurately differentiated patients with migraine from controls, with 84.9% accuracy (Figure 1). The right temporal pole was involved in 13 of the 15 correlations.
Henze-Penrose Divergence Between Patients With Migraine and Healthy Controls
Values range from 0.5 (subject groups cannot be separated) to 1 (subject groups are completely separable).
Adapted from Schwedt TJ et al. Temporal lobe cortical thickness correlations differentiate the migraine brain from the healthy brain. PLoS One. 2015;10:e0116687. doi:10.1371/journal.pone.0116687
Dr Schwedt concluded that measurements of cortical thickness are promising as a biomarker for migraine. Ongoing studies are assessing combinations of different structural measures, migraine subclassification, differentiating migraine from other headache disorders, and addition of functional data.
Higher Risk of Recurrent ICH With Inadequate BP Control After Lobar and Nonlobar ICH
ICH survivors have a high risk for recurrent ICH of even greater severity. Elevated BP is associated with increased risk of recurrent nonlobar ICH. Available evidence suggests that BP reduction reduces the risk of recurrent ICH but the optimal degree of reduction is not clear. BP reduction in elderly ICH survivors may increase risk of ischemic stroke, ischemic heart disease, renal failure, and syncope. The aim of this study, presented by Christopher D. Anderson, MD, Massachusetts General Hospital, Boston, Massachusetts, USA, was to characterize the relationship between elevated BP after index lobar and nonlobar ICH and risk of ICH recurrence.
This single-center observational study included 1145 ICH survivors (505 lobar, 640 nonlobar). Ambulatory BP was measured at 3, 6, 9, and 12 months after ICH and every 6 months thereafter. The end point was recurrent ICH (lobar vs nonlobar). During follow-up, recurrent ICH occurred in 102 patients with initial lobar ICH and 42 patients with initial nonlobar ICH. Inadequate BP control was significantly associated with ICH recurrence in lobar ICH (HR, 3.53; 95% CI, 1.65 to 7.54; P = .001) and nonlobar ICH (HR, 4.23; 95% CI, 1.02 to 17.52; P = .048) survivors. All stages of hypertension, including prehypertension, were significantly (P < .05) associated with ICH recurrence in both lobar and nonlobar ICH survivors, except for stage 2 hypertension in nonlobar ICH survivors.
There was evidence of a dose-response relationship, with a linear association between BP values and recurrent ICH.
Elevated BP after both lobar and nonlobar ICH is associated with increased risk of recurrent ICH. If these results are confirmed, current recommendations for BP control after ICH may be inadequate. Clinical trials are needed to determine the optimal degree of BP reduction for prevention of recurrent ICH and minimization of side effects.
Convergent Molecular Pathways May Lead to Targeted Therapies for Autism
Autism is a neuropsychiatric syndrome characterized by deficits in social communication and interactions and restrictive, repetitive behavior. Daniel H. Geschwind, MD, PhD, University of California, Los Angeles School of Medicine, Los Angeles, California, USA, discussed progress in the past 2 decades of genetic research in autism spectrum disorders (ASDs) and explored the potential for using autism risk genes to develop targeted treatments.
Two large, open resources for ASD genetics are available. The Autism Genetic Resource Exchange, founded in 1998, includes > 1500 families and 6000 individuals. The Simons Simplex Collection comprises 2700 families and > 10 000 individuals. Both resources have large-scale genetic and phenotypic data. Twelve major linkage studies (1998-2009) and genome-wide association studies have revealed the genetic heterogeneity of ASDs [Abrahams BS, Geschwind DH. Nat Rev Genet. 2008].
Major advances were made in 2012, said Dr Geschwind, when 4 groups published results of exome sequencing in almost 1000 patients with ASDs. Several dozen mutations were identified, including several observed more than once; however, most appear to increase ASDs risk rather than cause ASDs. The most frequent genes (CHD8, DYRK1A, GRIN2B, TBR1, PTEN, and TBL1XR1) account for < 1% of cases. Based on these findings, Dr Geschwind estimated that > 500 genes contribute to ASDs. These results were confirmed in a study on > 2500 families [Iossifov I et al. Nature. 2014].
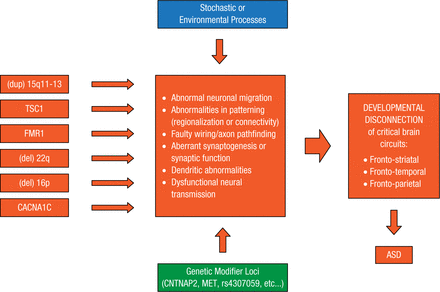
More than 100 genes contributing to ASDs have been identified. Development of targeted treatments for this extremely heterogeneic disorder might depend on using integrative genomics to find points of convergence among these genes at molecular and cellular levels and across brain circuits [Parikshak NN et al. Cell. 2013; Berg JM, Geschwind DH. Genome Biol. 2012]. A working model shows how major ASD risk genes might converge and lead to autism (Figure 2) [Geschwind DH. Trends Cogn Sci. 2011].
Levels of Convergence in ASD
ASD, autism spectrum disorder.
Reprinted from Geschwind DH. Genetics of autism spectrum disorders. Trends Cogn Sci. 2011;15:409-416. With permission from Elsevier Ltd.
While ASD risk is largely genetic, its etiology is multifactorial and heterogeneous. Even so, convergent evidence from transcriptional networks can be used to examine the neural systems basis of ASDs. Genetic findings are a starting point for creating in vitro and in vivo models, and systems biology approaches can be used to identify potential convergent molecular pathways.
- © 2015 SAGE Publications