Summary
The TEMSO and TOWER trials demonstrate that both doses of teriflunomide (7 mg and 14 mg) each significantly reduce the annualized relapse rates in patients with relapsing multiple sclerosis. Post hoc analyses of both trials indicate that only teriflunomide 14 mg significantly reduces the rate of all 5 markers of severe relapse in both studies.
- multiple sclerosis
- teriflunomide
- relapse
- TOWER
- TEMSO
- neurology clinical trials
- demyelinating diseases
Teriflunomide is a once-daily oral immunomodulating drug approved in the United States for the treatment of relapsing multiple sclerosis (RMS). Teriflunomide was tested in 2 phase 3 trials—TOWER [Confavreux C et al. Lancet Neurol. 2014] and TEMSO [O’Connor P et al. N Engl J Med. 2011]. Results from these 2 trials indicate that among people with RMS, teriflunomide 7 mg and 14 mg are both associated with significant reductions in the annualized relapse rate (ARR) compared with placebo.
Because severe MS relapses are associated with disability progression and substantial economic cost, an agent capable of reducing this type of relapse could reduce health care costs and improve patient outcomes. Richard Macdonell, MD, Austin Health, Victoria, Australia, highlighted data from post hoc analyses of TOWER and TEMSO regarding the effect of teriflunomide on severe relapses in a poster presentation.
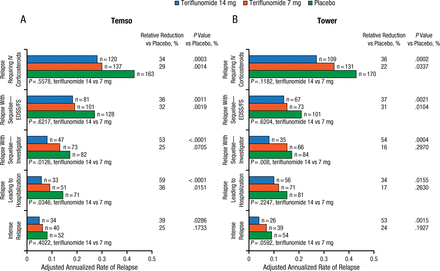
As there is no universal definition of what constitutes a severe relapse, these analyses examined the effect of teriflunomide on 5 surrogate markers: (1) relapses with sequelae defined by increase in Expanded Disability Status Scale score/Functional System score 30 days postrelapse; (2) relapses with investigator-defined sequelae; (3) severe relapses by the Panitch definition, which is based on specified increases in Expanded Disability Status Scale and Functional System score used in the EVIDENCE trial in 2002; (4) relapses leading to hospitalization; and (5) relapses associated with increased use of health care resources, including those requiring intravenous corticosteroid treatment. All analyses were performed on the modified intent-to-treat population enrolled in each study (TEMSO, n = 1086; TOWER, n = 1165).
Compared with placebo, teriflunomide 14 mg significantly reduced annualized rates of all 5 indicators of severe relapse in both TEMSO and TOWER. Teriflunomide 7 mg significantly reduced the annualized relapse rates for several different indicators of relapse in both studies. The effects of teriflunomide 7 mg, 14 mg, and placebo on severe relapses in TEMSO and TOWER are shown in Figure 1.
Effects of Teriflunomide on Severe Relapses in TEMSO and TOWER
EDSS, Expanded Disability Status Scale; FS, Functional System; IV, intravenous.
Sources: O’Connor PW et al. J Neurol. 2013 and Miller AE et al. J Neurol. 2014.
Reproduced with permission from R Macdonell, MD.
Both teriflunomide doses showed similar and manageable safety profiles across the 2 studies. Adverse events emerging more often with teriflunomide than with placebo included elevations in alanine aminotransferase, as well as headache and diarrhea.
In summary, teriflunomide has shown consistent and significant efficacy on annualized relapse rates in both TEMSO and TOWER. Teriflunomide also reduced the rate of severe relapses, which may help reduce health care costs related to episodes of relapse, as well as improve patients’ quality of life.
- © 2015 SAGE Publications