Summary
Patients with mild Alzheimer’s disease enrolled in EXPEDITION EXT had already received 80 weeks of treatment with either solanezumab or placebo. Utilizing a unique approach to the delayed-start model, researchers report that cognitive and instrumental function outcomes in delayed-start patients did not catch up to those in early-start patients, supporting the benefit of initiating treatment as early as possible.
- solanezumab
- Alzheimer’s disease
- delayed-start
- EXPEDITION EXT
- NCT01127633
- dementias
- neurology clinical trials
Alzheimer’s disease (AD) is a progressive neurological condition characterized pathologically by beta-amyloid plaques and neuronal loss. Because the monoclonal antibody solanezumab binds to amyloid, researchers have hypothesized that it would enhance the clearance of amyloid from the brain. However, published results from 2 phase 3 trials (EXPEDITION and EXPEDITION 2) reported that solanezumab produced no significant improvement in cognitive or functional ability in patients with mild-to-moderate AD [Doody RS et al. N Engl J Med. 2014]. A prespecified secondary analysis showed that there is a treatment signal on cognition in the patients with mild AD.
Patients who completed either of the EXPEDITION trials were eligible to enroll in the EXPEDITION EXT [NCT01127633], an open-label extension study, presented in a poster session by Hong Liu-Seifert, PhD, Eli Lilly and Company, Indianapolis, Indiana, USA. The goal of the extension trial was to continue monitoring the efficacy and safety of solanezumab out to 104 weeks, using a delayed-start design methodology. Dr Liu-Seifert noted that researchers can use this design to distinguish a treatment’s effect that alters the underlying disease pathology from an effect that only attenuates the symptoms of the disease. However, there are also methodologic challenges associated with previous applications of this design that limit its usage in clinical trials.
Dr Liu-Seifert then discussed a new modeling approach to the delayed-start design that was implemented for EXPEDITION EXT. This approach incorporated all of the randomized patients with mild AD only, from the time of initial randomization to the end of the delayed-start period, and merged them into a single model. Noninferiority was then tested using a proportional noninferiority margin that compared the treatment difference at the end of the delayed-start period with the treatment difference at the end of the placebo-controlled period.
In EXPEDITION EXT (delayed-start period), all patients received solanezumab. Patients randomized to the placebo group in the 2 EXPEDITION trials were considered the delayed-start group; those who had received solanezumab in the 2 trials were considered the early-start group.
Throughout EXPEDITION EXT, the study staff and patients remained blinded as to whether patients had received solanezumab or placebo in the previous trials.
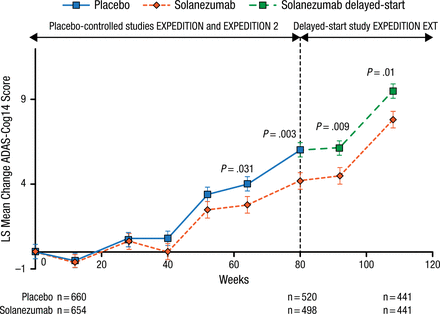
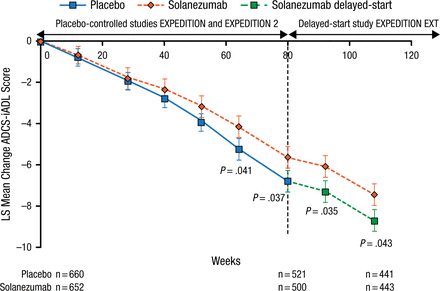
Results of the treatment differences on the 14-item cognitive subscale of the Alzheimer’s Disease Assessment Scale and the Alzheimer’s Disease Cooperative Study-instrumental activities of daily living at 28 weeks in EXPEDITION EXT are shown in Figures 1 and 2. The treatment differences between early-start and delayed-start patients remained statistically significant at 28 weeks for both measures. The noninferiority criterion, defined as the lower limit of 90% CI for Δ2 – 50% × Δ1 > 0, was met for both tests.
Results of ADAS-Cog14 in Patients With Mild AD at 28 Weeksa
AD, Alzheimer disease; ADAS-Cog14, 14-item cognitive subscale of the Alzheimer’s Disease Assessment Scale; LS, least squares.
aLower limit of 90% CI for Δ2 – 50% × Δ1 = 0.15.
Reproduced with permission from H Liu-Seifert, PhD.
Results of ADCS-iADL in Patients With Mild AD at 28 Weeksa
AD, Alzheimer disease; ADCS-iADL, Alzheimer’s Disease Cooperative Study-instrumental activities of daily living; LS, least squares.
aLower limit of 90% CI for Δ2 – 50% × Δ1 = 0.08.
Reproduced with permission from H Liu-Seifert, PhD.
In summary, 28-week data from EXPEDITION EXT suggest that patients with mild AD were able to sustain the cognitive effect noted in EXPEDITION and EXPEDITION 2. This effect was demonstrated in patients with mild AD and is probably consistent with a treatment effect that alters the underlying pathology of the disease.
- © 2015 SAGE Publications