Summary
Pasireotide LAR had a dose-proportional relationship with dose exposure over a 40- to 60-mg range. Efficacy end points of levels of growth hormone and IGF-1 were correlated with pasireotide exposure, and response rates were higher at the higher pasireotide dose.
- PAOLA
- NCT01137682
- pasireotide
- acromegaly
- growth hormone
- and insulin-like growth factor
- IGF-1
- somatostatin analogs
- endocrinology, diabetes & metabolism clinical trials
- pituitary gland disorders
Pasireotide long-acting release (LAR) showed a positive relationship between exposure and the efficacy end point of growth hormone (GH) and insulinlike growth factor (IGF-1) in patients with acromegaly in the phase 3 PAOLA study [NCT01137682]. In this pharmacokinetic (PK) and pharmacodynamic (PD) analysis, researcher Guoxiang Shen, PhD, Novartis Pharmaceuticals, East Hanover, New Jersey, USA, and colleagues found that treatment with pasireotide LAR has a positive risk-benefit profile for patients whose acromegaly is inadequately controlled by first-generation somatostatin analogs (SSAs).
Pasireotide, a multireceptor-targeted SSA, was recently approved for use in patients with acromegaly. Earlier results from the PAOLA study found that pasireotide was able to produce biochemical disease control in patients who were not responsive to other agents, with an efficacy of 15.4% with pasireotide LAR 40 mg and 20.0% at 60 mg vs 0% with octreotide LAR 30 mg or lanreotide Autogel 120 mg [Gadelha MR et al. Lancet Diabetes Endocrinol. 2014].
Patients in the 2 treatment arms received double-blind pasireotide LAR 40 or 60 mg every 28 days (n = 65 in each group). Patients in the open-label, active-control treatment arm (n = 68) received either octreotide LAR 30 mg or lanreotide Autogel 120 mg every 28 days.
Within the dose range of pasireotide LAR 40 to 60 mg that was evaluated, the dose-exposure relationship of pasireotide LAR was approximately dose proportional. The concentration of pasireotide reached steady state after 3 consecutive monthly injections. Interpatient PK variability was moderate to high.
The PK covariate of sex suggested that female patients would have approximately 51% higher steady-state trough concentration of pasireotide than male patients with the same age and equal baseline bilirubin. However, since efficacy and safety profiles were similar between female and male patients, this PK difference was not considered clinically meaningful.
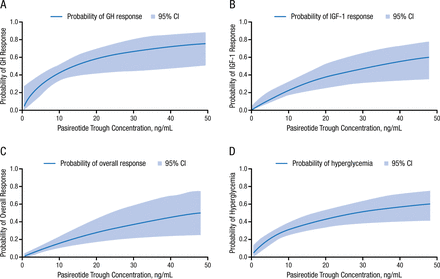
Levels of both GH and IGF-1 had a clear exposure-response relationship to pasireotide LAR concentration. The estimated maximum suppression of GH was 83.0%, which was relatively higher than the estimated maximum suppression of IGF-1 of 67.1%.
Increasing pasireotide trough concentration by 1.5-fold corresponds to dose increases from 40 to 60 mg. This 1.5-fold increase resulted in increased odds of GH responses by 44%, of IGF-1 responses by 51%, and of GH + IGF-1 responses by 54% (Figure 1). Also, a 1.5-fold increase in pasireotide concentration increased the odds of having hyperglycemia by 36%.
Pasireotide Trough Concentration Increases
GH, growth hormone; IGF-1, insulinlike growth factor.
Reprinted with permission from G Shen, PhD.
The change from baseline for both QTcF and QTcB had a relatively flat relationship with pasireotide concentrations based on data up to week 24, suggesting no clinically significant effect. Also, pasireotide exposure up to week 24 had no clinically significant effect on liver function tests, which included aspartate aminotransferase, alanine aminotransferase, total bilirubin, γ-glutamyltransferase, alkaline phosphatase, and albumin.
This PK/PD analysis found an approximately dose-proportional relationship for pasireotide LAR dose-exposure over the 40- to 60-mg range that was evaluated. Efficacy end points of GH and IGF-1 suppression had a positive relationship with pasireotide exposure, with greater response rates at the higher dose of 60 mg.
- © 2015 SAGE Publications