Summary
While good glycemic control is associated with preventing microvascular complications, its association with cardiovascular disease is far less straightforward. Key studies have yet to report a consistently positive effect of intensive glucose lowering on cardiovascular outcomes. For now, intensive glucose control should not be considered a strategy to prevent macrovascular complications.
- hyperglycemia
- cardiovascular
- type 2 diabetes mellitus
- macrovascular
- microvascular
- cardiology & cardiovascular medicine clinical trials
- endocrinology, diabetes & metabolism clinical trials
Silvio E. Inzucchi, MD, Yale University School of Medicine, New Haven, Connecticut, USA, presented the second of 2 Presidential Plenary sessions and focused on evolving views of glucose control and its effect on cardiovascular (CV) outcomes.
He began the session by reviewing the epidemiologic and pathophysiologic links between HbA1c and microvascular complications, as noted in the UKPDS 35 trial [Stratton IM et al. BMJ. 2000]. However, a definitive link between HbA1c and myocardial infarction (MI) and mortality remains more elusive and controversial [Emerging Risk Factors Collaboration. JAMA. 2014; Brewer N et al. Diabetes Care. 2008].
According to Dr Inzucchi, this lack of an association is probably due to the fact that atherosclerosis occurs at the macrovascular level, which is influenced by a variety of factors, including genetics, blood lipids, inflammation, obesity, blood pressure (BP), and smoking, as well as hyperglycemia and probably insulin resistance. Microvascular complications, however, occur in a single epithelial layer within the capillaries, where the predominant risk factors are hyperglycemia, BP, and genetics.
Dr Inzucchi went on to highlight results from the 5 major clinical trials that have investigated the impact of intensive therapy for diabetes on CV outcomes: (1) UKPDS 33 [UK Prospective Diabetes Study. Lancet. 1998], (2) DCCT/EDIC [Holman RR et al. N Engl J Med. 2008; The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993], (3) ACCORD [Action to Control Cardiovascular Risk in Diabetes Study Group. N Engl J Med. 2008], (4) ADVANCE [Advance Collaborative Group. N Engl J Med. 2008], and (5) VADT [Duckworth W et al. N Engl J Med. 2009].
Dr Inzucchi emphasized that among all these aforementioned studies, microvascular complications were improved, but a neutral effect was seen on mortality and CV events. The exceptions are the trials with long-term components (UKPDS and DCCT/EDIC), where there was an improvement in both mortality and CV events, and the ACCORD trial, where there was an increase in mortality. Dr Inzucchi cautioned that the increase in mortality seen in ACCORD was likely driven by an older group of patients with a high risk of overt atherosclerosis at baseline. Further, data from the VADT study suggested that intensive glucose lowering reduced CV events only in those with no or little preexisting atherosclerosis in coronary arteries [Reaven PD et al. Diabetes. 2009]. Aside from these 5 trials, the ORIGIN Trial [ORIGIN Trial Investigators. N Engl J Med. 2012] showed that insulin glargine had a neutral effect on CV outcomes in patients with only mild hyperglycemia over a median follow-up of 6.2 years. Dr Inzucchi also observed that, based on meta-analyses, if there is a CV benefit from glucose lowering, it is on the order of a 15% relative risk reduction.
Dr Inzucchi then moved on to discuss CV issues associated with various therapies for type 2 diabetes mellitus (T2DM; Table 1). Metformin probably provides some CV advantage over diet and possibly sulfonylurea drugs. The TZD pioglitazone appeared to reduce major adverse CV events in 1 trial, but these data need to be confirmed. This specific class of insulin-sensitizing drugs remains highly controversial.
Cardiovascular Issues Associated With Therapies for T2DM
In 2008, the US Food and Drug Administration (FDA) issued an industry guidance that mandated manufacturers of diabetes medications to demonstrate that their therapy would not result in an unacceptable increase in CV risk [US Department of Health and Human Services. Guidance for Industry. 2008]. This guidance was issued as a direct result of the controversy surrounding rosiglitazone and its purported association with poor CV outcomes. Dr Inzucchi reviewed the main points of the guidance, which mandates that manufacturers implement additional pre- and postmarketing CV outcome trials (CVOTs), using the following metrics:
-
Premarketing analyses: HR for major adverse CV events (MACEs) to include an upper limit of the 95% CI < 1.8
-
Postmarketing analyses: HR for MACEs to include an upper limit of the 95% CI < 1.3
-
Meta-analysis strategy using phase 2/3 data
-
Blinded central adjudication of CV events in phase 2/3
-
Inclusion of high-risk patients
-
Minimum exposure of 2 years
-
Approximately 15 000 patient years
Dr Inzucchi then discussed the published results from 2 of these CVOTs—SAVOR-TIMI 53 [Scirica BM et al. N Engl J Med. 2013] and EXAMINE [White WB et al. N Engl J Med. 2013]. SAVOR-TIMI 53 followed > 16 000 patients who had been randomized to either saxagliptin or placebo over a median of 2.1 years with a primary end point of cardiovascular death, nonfatal myocardial infarction or nonfatal ischemic stroke (saxagliptin, n = 613; placebo, n = 609). Although there was neither an increase nor a decrease in ischemic results among patients in the saxagliptin arm, the rate of hospitalization for heart failure was significantly increased (P = .007). In the EXAMINE trial, 5380 patients who had T2DM and were hospitalized for either an MI or unstable angina were randomly assigned to alogliptin (n = 2701) or placebo (n = 2679). Patients were followed for up to 40 months (median, 18 months). There was no significant difference between the groups in mortality from CV causes, nonfatal MI, and nonfatal stroke (P Noninferiority < .001).
A number of other trials are ongoing, with results due over the next few years (Table 2). Dr Inzucchi emphasized that although these preliminary studies may report effects on CV markers and surrogates, these effects do not necessarily predict what will happen to patients in terms of actual clinical events in the CVOTs. In addition, while the drugs may present no CV safety signals during these trials, it may be overly optimistic to think that they will show any differences in effectiveness on CV end points over a period of just 2 to 4 years. And because the FDA has demanded the recruitment of high-risk patients into these CVOTs, there is some concern that the presence of advanced CV disease and an underlying predilection for further overt macrovascular complications will outweigh any possible CV benefits of a glucose-lowering drug.
Ongoing Cardiovascular Trials for Diabetes Drugs (Noninsulin)
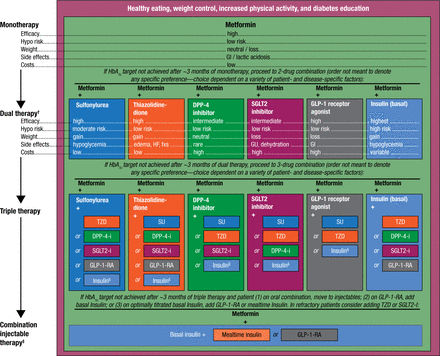
Dr Inzucchi then reviewed the updated 2015 American Diabetes Association/European Association for the Study of Diabetes treatment algorithm for managing hypertension in T2DM (Figure 1). He noted that if any of the compounds included in 1 of the ongoing CVOTs were to show a CV benefit, the current treatment algorithm would likely be updated to reflect that benefit.
General Recommendations for Antihyperglycemic Therapy, T2DM
GLP-1 RA, glucagon-like peptide 1 receptor agonist; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SGLT2i, sodium-glucose cotransporter-2 inhibitor; SU, sulfonylurea; T2DM, type 2 diabetes mellitus; TZD, thiazolidinedione.
†Consider initial therapy at this stage when HbA1c is 9% (75 mmol/mol).
‡Consider initial therapy at this stage when blood glucose is 300–350 mg/dL (16.7–19.4 mmol/L) and/or HbA1c 10–12% (86–108 mmol/mol), especially if patient is symptomatic or if catabolic features (weight loss, ketosis) are present, in which case basal insulin 1 mealtime insulin is the preferred initial regimen.
§Usually a basal insulin (eg, NPH, glargine, detemir, degludec).
Reproduced from Inzucchi SE et al. Management of Hyperglycemia in Type 2 Diabetes 2015: A Patient Centered Approach, Diabetes, 38, 2015, Copyright and all rights reserved. Material from this publication has been used with the permission of American Diabetes Association.
In closing, Dr Inzucchi emphasized that although diabetes confers additional CV disease risk, glycemic control itself appears to only modestly reduce nonfatal MI. Any benefit from glycemic control will likely have to accrue over many years and may be negated by progressive atherosclerosis. The ideal glucose-lowering agent will reduce CV events as well as maintain glycemic control. For now, however, glycemic control should be used mainly to prevent microvascular complications, while control of lipids and BP is the best means for preventing macrovascular complications.
- © 2015 SAGE Publications